
How Does a Vacuum Cooler Make Vegetables Cold?
It seems almost magical: you place warm vegetables in a steel chamber, and just 20-30 minutes later, they emerge perfectly chilled to their core. There’s no ice and no freezing air. How is this rapid, deep cooling even possible?
Understanding the science behind a new technology is crucial before you invest in it. If the process seems like a "black box," it’s hard to trust that it will be reliable, safe for your produce, and effective for your business. You need to know that it’s based on solid, predictable principles.
Vacuum coolers work by using a powerful vacuum pump to rapidly lower the air pressure inside a sealed chamber. This low pressure forces the water on the surface of the vegetables to boil at a very low temperature (e.g., 2°C). This "boiling" creates an evaporative cooling effect that pulls heat out of the produce quickly and uniformly.
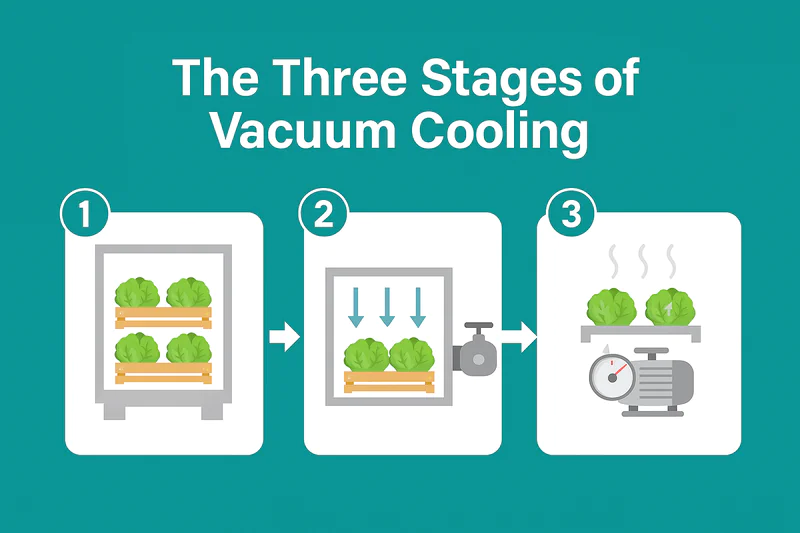
As someone who builds these machines, I love explaining the physics because it’s so elegant and effective. It’s a natural process that we have simply optimized with modern technology. In this article, I will walk you through the entire process, step by step, and explain the science behind each stage. Let’s open the door and look inside.
Step 1: Why Does Lowering the Pressure Matter?
The first step in any vacuum cooling cycle is sealing the chamber door and turning on the vacuum pump. You hear the pump start, and you see the pressure gauge on the control panel begin to fall rapidly. But what is actually happening physically?
If you don’t understand the relationship between pressure and the boiling point of water, the rest of the process won’t make sense. It’s easy to assume that the vacuum itself is cold, but that’s not true. The vacuum is just the trigger for the cooling effect.
Lowering the air pressure inside the chamber is the key that unlocks low-temperature boiling. At normal sea-level pressure, water boils at 100°C (212°F). But in a near-perfect vacuum, water will boil at just 2°C (36°F). This fundamental principle of physics is called Boyle’s Law.
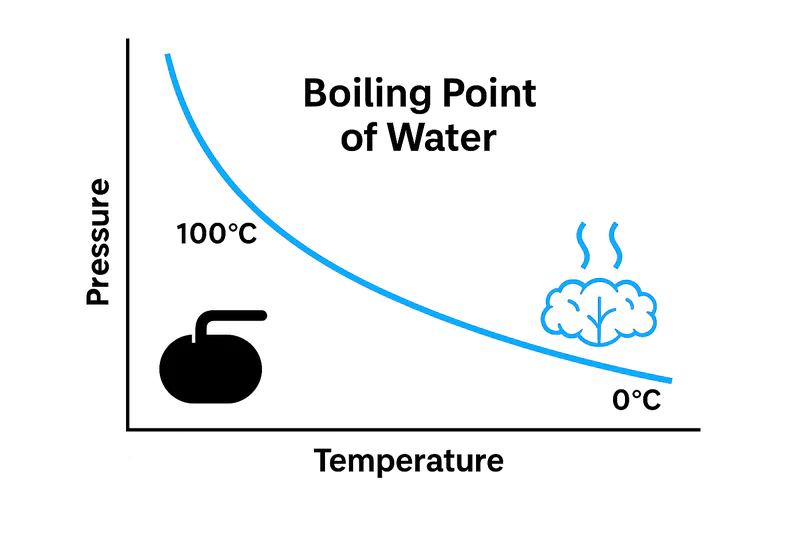
The Physics of Boyle’s Law1
This relationship is the heart of vacuum cooling. For a technical buyer like Carlos, understanding this principle is key to trusting the technology. It’s not magic; it’s applied thermodynamics.
Boiling Without Heat
We are all taught that water boils at 100°C. But that’s only true at standard atmospheric pressure (101.3 kPa). Boiling is the process where a liquid turns into a gas. For a water molecule to escape from the liquid and become vapor, it must have enough energy to overcome the pressure of the air pushing down on the water’s surface. If you reduce that air pressure, the molecules need far less energy to escape. Inside our vacuum chamber2, we use a powerful pump to remove over 99% of the air, dropping the pressure to a tiny fraction of normal. At this extremely low pressure, the water molecules on the surface of the lettuce or broccoli have more than enough energy to "boil" and turn into vapor, even when the vegetable is at a cool room temperature.
Setting the Target Temperature
This principle gives us precise control. The control system of the vacuum cooler is programmed to pull the vacuum down to a specific pressure level and hold it there. This pressure level corresponds to a specific boiling point for water. If our target is to cool the lettuce to 2°C, the PLC will run the vacuum pump until the pressure in the chamber reaches 0.7 kPa, the pressure at which water boils at 2°C. This means the cooling process cannot go below the target temperature, making it impossible to accidentally freeze the produce.
| Atmospheric Condition | Air Pressure | Water Boiling Point |
|---|---|---|
| Normal (Sea Level) | ~101 kPa | 100°C (212°F) |
| High Altitude (Everest) | ~34 kPa | 71°C (160°F) |
| Inside Vacuum Cooler | < 1 kPa | ~2°C (36°F) |
Step 2: How Does Evaporation Create the Cooling Effect?
Once the pressure in the chamber is low enough for water to boil at a low temperature, the second stage begins. This is where the actual cooling happens. You would see a large volume of water vapor being generated inside the chamber.
It might seem counterintuitive. How can "boiling" make something cold? We associate boiling with heat, like a hot kettle. But in this context, it’s the very same process that cools your body when you sweat.
This low-temperature boiling is a phase change from liquid to gas, which is called evaporation. This process requires a tremendous amount of energy, known as the "latent heat of vaporization." The water molecules take this energy from the only available source: the vegetables themselves, rapidly pulling the heat out of them.
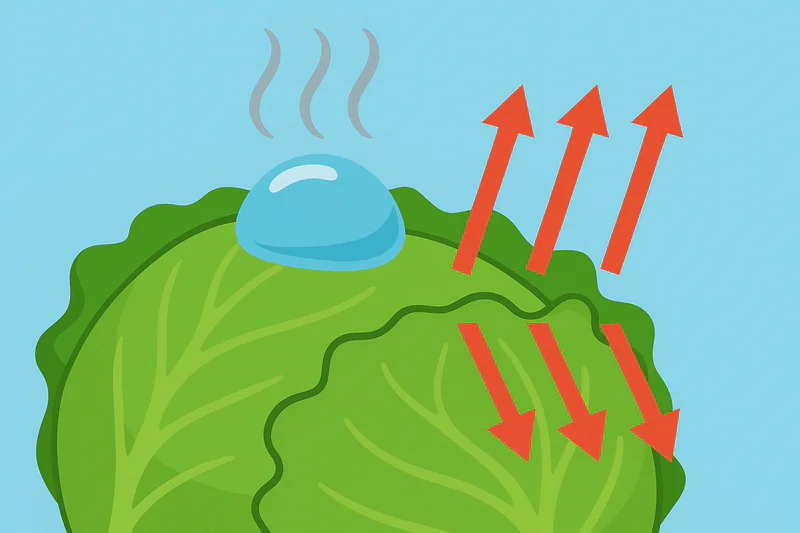
The Power of Latent Heat
For a procurement manager like Sophia, who is responsible for the final quality3 of her food products, the quality of the cooling is just as important as the speed. The evaporative process provides a unique benefit.
Deep and Uniform Cooling
Every gram of water that evaporates from the produce removes a fixed amount of heat energy. Because vegetables like lettuce are more than 95% water, they have a lot of moisture to give. This evaporation happens on all surfaces of the vegetable at the same time—the outside, the inside of the leaves, and even down to the core. This is why vacuum cooling4 is so uniform. Unlike a traditional cold room, which cools from the outside-in and can take many hours to cool the core, vacuum cooling pulls heat from the entire product simultaneously. This results in a product that is evenly chilled through and through, with no warm spots in the middle of the pallet.
How Much Water is Lost?
This is a common and important question. The process relies on evaporating water, so some moisture loss5 is required. However, the amount is very small and precisely calculable. As a rule of thumb, for every 6°C of cooling, the product will lose approximately 1% of its weight in water. So, to cool lettuce from 28°C down to 4°C (a 24°C drop), the moisture loss would be about 4%. For many leafy greens, this is an acceptable trade-off for the massive gain in cooling speed and shelf life. For produce that is more sensitive to water loss, like mushrooms or berries, our machines have a "hydro-vac" feature that adds a fine mist of clean water during the cycle to compensate.
| Cooling Method | Time to Cool (Core) | Cooling Uniformity | How it Works |
|---|---|---|---|
| Vacuum Cooling | 20-30 minutes | Excellent (Uniform) | Evaporation pulls heat from the entire product at once. |
| Forced-Air Cooling | 3-6 hours | Good | Cold air is blown over the product. |
| Cold Room | 12-24 hours | Poor (Outside-in) | Still, cold air slowly chills the surface. |
Step 3: Where Does All the Heat Go?
The first two steps explain how heat is removed from the vegetables and turned into a massive volume of water vapor. But that energy can’t just disappear. The final step is to remove this heat and vapor from the system permanently.
If this hot vapor isn’t dealt with, the chamber pressure would rise again, and the cooling process would stop. The system would be choked with its own exhaust. This is the job of the refrigeration system, also known as the "cold trap."
The hot water vapor is drawn over a large, freezing-cold radiator coil (the evaporator or cold trap) located between the chamber and the vacuum pump. The vapor instantly condenses back into ice on this coil, effectively trapping the heat. The powerful refrigeration system then pumps this captured heat out of the building.
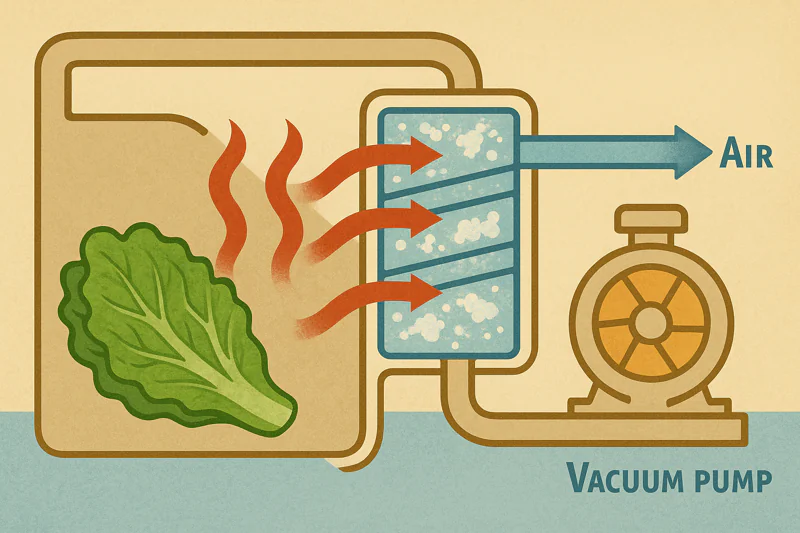
The Unsung Hero of the System
For a professional buyer like Norman, who is sensitive to quality and reliability, the design of the refrigeration system is critical. A system with an undersized cold trap is a common failure point in cheaper machines, leading to poor performance and high maintenance costs.
Capturing the Vapor
The evaporator coil is the workhorse of the system. It is cooled by a powerful refrigeration circuit, typically using a high-quality Bitzer compressor6. As the warm, moist vapor created during the cooling cycle passes over these coils, which are well below freezing, it changes phase again—from a gas directly into a solid (ice). This process is called deposition. It effectively removes the vapor from the system, allowing the vacuum pump to maintain the low pressure needed for cooling.
Defrosting the System
After several cooling cycles, a thick layer of ice will build up on these coils. This is the water that was removed from the produce. A well-designed machine includes an automatic defrost cycle7. At the push of a button, the system reverses, flowing hot refrigerant gas through the coils to quickly melt the ice. The water drains away, and the machine is ready for the next shift. Making this process fast and efficient is a key part of our design philosophy, ensuring maximum uptime for our customers.
Conclusion
The process of vacuum cooling is a perfect blend of physics and engineering. By understanding these three simple steps—lowering pressure, evaporating water, and trapping the heat—you can see that it is a highly controlled, predictable, and incredibly effective way to chill produce.
-
Understanding Boyle’s Law is crucial for grasping how vacuum cooling works, enhancing your knowledge of thermodynamics. ↩
-
Exploring the function of a vacuum chamber can provide insights into innovative food preservation techniques and their benefits. ↩
-
Understanding the significance of quality in cooling can enhance your food preservation strategies. ↩
-
Explore the mechanics of vacuum cooling to improve your food storage and shelf life. ↩
-
Learn about moisture loss effects to make informed decisions on food quality and preservation. ↩
-
Explore this link to understand the technology behind Bitzer compressors, crucial for efficient refrigeration systems. ↩
-
Learn about the automatic defrost cycle to see how it enhances efficiency and performance in refrigeration. ↩

Mila
You May Also Like

How Do You Handle the Peak Season Vegetable Rush?
The harvest season is here. Your fields are full of beautiful produce, but now you face the biggest challenge: a

Can You Vacuum Cool Vegetables After They Are Packaged?
You’ve just packed bags of beautiful, fresh-cut salad mix. But the product is still warm from processing and washing. This

How Do You Perfectly Cool Leafy Greens Without Damaging Them?
You’ve invested in a vacuum cooler to protect your leafy greens, but the results aren’t always perfect. Sometimes the lettuce

Will Your Vegetables Work in a Vacuum Cooler?
You’ve harvested a perfect crop, but the clock is ticking. Every minute of field heat is degrading the quality, reducing

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Are You Gambling with Your Export-Quality Vegetables?
You’ve grown a perfect crop, meeting every standard for size, color, and taste. Now comes the biggest challenge: shipping it

Is Your Cold Chain Broken Before It Even Starts?
Your company has invested millions in refrigerated trucks, state-of-the-art warehouses, and sophisticated inventory systems—a world-class cold chain. Yet, you’re still

Is Vacuum Cooling a Non-Negotiable Tool for Organic Growers?
As an organic producer, you’ve committed to a higher standard. Your customers pay a premium for vegetables that are not

Can Small Farms Actually Afford a Vacuum Cooler?
You’ve poured your heart into your farm, producing the highest quality vegetables. But as soon as they’re picked, the summer
How Do You Customize a Vacuum Cooling Cycle for Different Vegetables?
You’ve invested in a powerful vacuum cooler, expecting it to be a simple "set it and forget it" solution. But
