How Does Physics Allow Vacuum Cooling to Work?
You see a vacuum cooler in action and it seems like magic. A trolley of piping hot bread, fresh from the oven, goes into a stainless steel box. Three minutes later, it comes out perfectly cooled, with a crispier crust and ready to be packaged. How is this possible?
This process defies everything you know about cooling. It’s too fast. It feels unnatural. You worry that some strange, aggressive process is happening inside that box that could be harming your product. The lack of understanding makes you skeptical, and you hesitate to explore a technology that could fundamentally improve your entire business.
The science of vacuum cooling is based on a fundamental law of physics: the boiling point of water decreases as air pressure decreases. By creating a strong vacuum, the machine forces the water inside your bread to boil at a very low temperature (around 30°C), and this rapid evaporation is what pulls the heat out of the product.
This isn’t magic, and it isn’t a new discovery. It’s a principle that physicists have understood for centuries, and it’s the same reason why it’s hard to brew a good cup of tea on top of a high mountain. As a manufacturer, my job is to take this powerful scientific principle and engineer it into a reliable, controllable machine for your bakery. Let’s break down the science, step by step.
Why Does Water Boil at a Low Temperature in a Vacuum?
When you think of "boiling," you think of a pot of water on a stove at 100°C (212°F). The idea of boiling your bread sounds destructive because you associate it with intense heat. This is the first and most important concept we need to clarify.
You see the term "boiling" and you immediately picture something that would burn or ruin a delicate baked good. This mental image is the biggest barrier to understanding the process. You think, "How can boiling something possibly make it cooler?" It’s a completely fair question.
In a vacuum, water boils at a low temperature because there is no air pressure pushing down on its surface. This "cold boil" is a gentle phase transition from liquid to gas that happens without high heat, making it perfect for rapidly cooling sensitive products like bread.
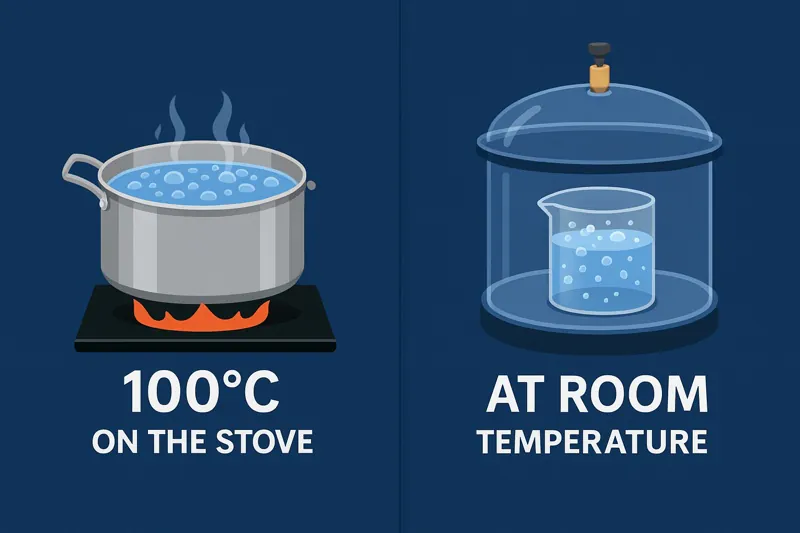
Understanding Pressure and Its Effect
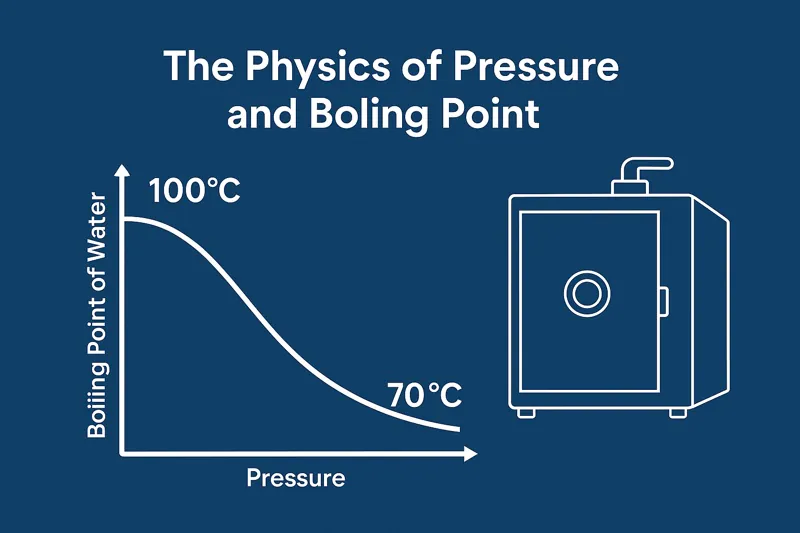
To understand this, you have to think about what air pressure really is. At sea level, we are all living at the bottom of an ocean of air. The weight of all that air above us creates a pressure of about 101 kilopascals (kPa). For water to boil, its molecules need enough energy to escape the liquid and push against that blanket of air. At sea level, this requires heating the water to 100°C.
Now, imagine you are on top of Mount Everest. The column of air above you is much shorter, so the air pressure is only about 33 kPa. With less pressure pushing down on it, the water needs far less energy to escape. On Everest, water boils at only 70°C (158°F).
A vacuum cooler1 takes this principle to the extreme. A powerful vacuum pump’s job is to remove almost all the air from the sealed chamber. Inside our machines, we lower the pressure to less than 6 kPa. At this incredibly low pressure, water no longer needs to be hot to boil. It will boil vigorously at just 35°C (95°F) or even cooler. This is not a heat-driven process; it is a pressure-driven one.
| Location / Condition | Atmospheric Pressure (Approx.) | Boiling Point of Water |
|---|---|---|
| Sea Level (Standard) | 101.3 kPa | 100°C / 212°F |
| Mexico City (High Alt.) | 78 kPa | 93°C / 199°F |
| Mount Everest Peak | 33 kPa | 70°C / 158°F |
| Allcold Vacuum Cooler | < 6 kPa | < 35°C / < 95°F |
So, when we say the bread "boils," we are not cooking it further. We are simply creating an environment where the water inside it can effortlessly turn into vapor at a cool, room-like temperature.
How Does Evaporation Actually Remove Heat from Bread?
You understand that the water is boiling at a low temperature, but the next logical question is: "How does that actually make the bread cold?" The vacuum itself isn’t cold. The chamber walls are just room temperature steel. Where does the cooling power come from?
The connection between evaporation and cooling might not be immediately obvious. It seems like a passive side effect, but in reality, it is the entire engine of the cooling process. Without understanding this energy transfer, the speed of vacuum cooling remains a mystery.
The process of evaporation is highly endothermic, meaning it requires a large amount of energy to happen. In a vacuum cooler, the only source for this energy is the bread itself. As water evaporates from the loaf, it forcefully pulls heat energy with it, cooling the bread from the inside out.
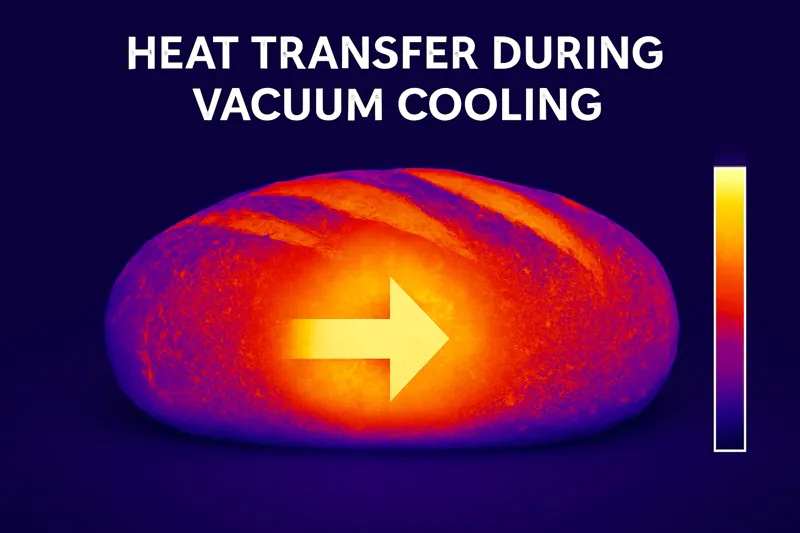
The Power of Latent Heat
This phenomenon is called "latent heat of vaporization2." You experience it every day. When you get out of a swimming pool, you feel cold as the water evaporates from your skin. The evaporating water is pulling heat energy directly from your body. Vacuum cooling weaponizes this simple principle.
Here is the crucial part: it takes a massive amount of energy to change water from a liquid to a gas (vapor). To evaporate just 1 gram of water, it takes about 2,260 joules of energy. When you place a trolley with 100kg of hot bread in the chamber, that bread contains several kilograms of water. As the vacuum pump lowers the pressure, this water starts to evaporate. To do so, it must find that energy. The only place it can get it from is the bread and the remaining water. This mass evaporation triggers a massive and instantaneous drop in the bread’s temperature.
Because the water is distributed throughout the entire loaf, this cooling happens simultaneously everywhere—at the core and at the crust. This is what we mean by "inside-out" cooling. It’s fundamentally different from traditional cooling3, which is an "outside-in" process.
| Cooling Method | Mechanism | Direction of Cooling | Speed | Effect on Product Core |
|---|---|---|---|---|
| Traditional Cooling | Convection (Air moving over the surface). | Outside -> In | 2 – 3 hours | Core remains hot for a very long time. |
| Vacuum Cooling | Evaporation (Latent Heat Transfer) | Inside -> Out | 3 – 6 minutes | Core cools as fast as the surface. |
This is why vacuum cooling4 is so uniquely effective for dense products. A traditional cooling rack might cool the surface of a meat pie, but the dense, hot filling in the center can stay dangerously hot for hours. With vacuum cooling, the heat is pulled from the core of the filling just as quickly as it is from the crust.
What is Happening to the Bread’s Structure During Cooling?
You are a baker. You care deeply about the quality of your product. The science is interesting, but your biggest concern is the final result. Will this powerful process damage the delicate crumb structure? Will it dry out the bread? Will the crust survive?
These are valid, professional concerns. You have spent years perfecting your recipes and baking process. The cooling step, as slow as it is, is a known quantity. Introducing such a radical change is frightening. You need assurance that the science is working for your product, not against it.
Vacuum cooling has a profound and positive impact on the bread’s structure. The rapid cooling immediately halts the starch gelatinization process, strengthens the crumb, sets a crispy crust by removing surface moisture, and dramatically extends shelf life by quickly passing through the microbial danger zone.
The Science of Quality Improvement
Let’s look at what’s happening to the bread on a structural level. This is where a technical owner like Carlos gets excited, and a quality-focused manager like Sophia sees the direct food safety benefits.
- "Gelatinization Stop5": During baking, the starches in your flour absorb water and swell up, creating the soft crumb of your bread. This is called gelatinization. When bread cools slowly, this process can partially reverse, the structure can weaken, and water can start to move around. Vacuum cooling is so fast that it acts like a "shock," locking the swollen starch structure in its perfect, freshly-baked state. This results in a loaf with better volume, a stronger slice, and a more stable crumb.
- Crispy Crust Formation6: Why does a crust go soggy during traditional cooling? Because moisture from the hot interior of the bread slowly migrates outwards and gets absorbed by the crust. Vacuum cooling turns this problem into a solution. The process actively pulls a small amount of moisture (1-2%) from the entire loaf and expels it as vapor. This includes the surface moisture that would normally cause sogginess, leaving behind an incredibly dry, stable, and crispy crust.
- Extended Shelf Life7 (The Microbial Impact): This is a huge benefit for anyone selling packaged goods. The temperature range between 60°C (140°F) and 10°C (50°F) is the "danger zone" where mold spores and bacteria multiply fastest. Traditional cooling methods can leave your bread in this zone for over an hour. A vacuum cooler passes through this entire zone in under five minutes. By preventing this initial bloom of microbial activity, you can add days to the product’s natural shelf life without using preservatives. This is a critical point for meeting HACCP food safety plans8.
| Scientific Effect | Impact on Baked Good | Benefit for the Bakery |
|---|---|---|
| Gelatinization Stop | Strengthens crumb structure, improves volume. | More stable product, better for slicing and sandwiches. |
| Rapid Evaporation | Dries the surface, preventing moisture migration. | Creates a wonderfully crispy and long-lasting crust. |
| Fast Temperature Drop | Passes through the "danger zone" in minutes. | Naturally extends shelf life by 3-7 days, reduces waste. |
How Does a Machine Control This Scientific Process?
The physics is elegant, but a bakery is an industrial environment. You can’t have a physicist running calculations for every batch of croissants. The process needs to be reliable, repeatable, and easy for your staff to operate. How do you tame this powerful science?
You might worry that such a sophisticated process requires an equally sophisticated operator. You have a busy production floor with staff who need to focus on baking, not on managing complex machinery. The technology is useless if it is not practical and robust.
A modern bakery vacuum cooler uses a precisely integrated system of three key components—a vacuum pump, a refrigeration system, and a PLC controller—to automate and control the scientific process. This allows you to get perfect, repeatable results for any product with the push of a button.
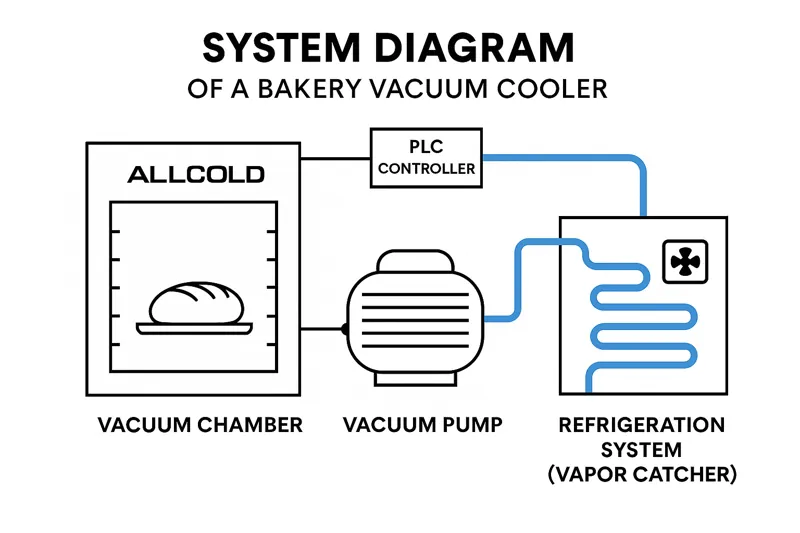
The Engineering Behind the Science
As an engineer, this is the part I love. We take these scientific principles and build a machine that masters them. A buyer like Norman, who is focused on quality, will recognize the importance of the brands we use for these core components.
- The Vacuum Pump (The "Lungs"): This is the heart of the system. We use high-quality German brands like Busch or Leybold. Its job is to remove the air from the sealed chamber to lower the pressure. The size of the pump and the speed at which it works determine how fast the pressure drops, which is a key variable in the cooling "recipe."
- The Refrigeration System (The "Vapor Catcher"): This is a critical but often overlooked component. As the water evaporates from the bread, it fills the chamber as water vapor. If this vapor went directly into the oil-sealed vacuum pump, it would contaminate the oil and destroy the pump. To prevent this, we have a set of extremely cold refrigeration coils inside the system. The hot water vapor hits these cold coils and instantly condenses back into water or freezes into ice. This traps the water, protecting the pump and allowing the vacuum to be maintained. We use robust compressors from brands like Bitzer to ensure this system is powerful and reliable.
- The PLC Controller (The "Brain"): This is where the magic becomes controllable. We use advanced Siemens PLC controllers with a simple touch screen interface. We work with you to program specific "recipes" for each of your products. A recipe for a delicate cake might lower the pressure slowly in stages to prevent collapse. A recipe for a dense rye bread might use a very fast and aggressive pressure drop. This brain controls the pump and the refrigeration system, ensuring you get the exact same perfect cooling curve every single time.
| Component | Brand Example | Function in the Scientific Process |
|---|---|---|
| Vacuum Pump | Busch / Leybold | Creates the low-pressure environment required to initiate the low-temperature boiling. |
| Refrigeration System | Bitzer | Captures the water vapor by condensation, protecting the pump and sustaining the vacuum. |
| PLC Controller | Siemens | Automates and controls the entire process, managing pressure curves and cycle times for different products. |
Conclusion
The science of vacuum cooling is not magic. It is a brilliant application of the laws of physics—pressure, evaporation, and energy transfer. By understanding this science, you can see that it is a gentle, controllable, and incredibly effective process that enhances, rather than harms, your beautifully crafted baked goods.
-
Exploring the mechanics of vacuum coolers can provide insights into innovative cooking techniques and food preservation. ↩
-
Understanding latent heat of vaporization is crucial for grasping how energy transfer works in various processes, including cooking and cooling. ↩
-
Understanding the limitations of traditional cooling can help you appreciate the innovations in cooling technology and their benefits. ↩
-
Exploring vacuum cooling can reveal its advantages in food preservation and efficiency, making it a game-changer in the food industry. ↩
-
Understanding this process can enhance your baking skills and improve bread quality. ↩
-
Explore how crust formation impacts texture and taste, crucial for any baker. ↩
-
Learn how to prolong freshness and reduce waste in your bakery products. ↩
-
Discover essential food safety practices that can benefit your bakery operations. ↩

Mila
You May Also Like

When Should You NOT Use Vacuum Cooling for Your Bakery?
Are you tired of waiting hours for your bread to cool down while your profits evaporate? You might think vacuum

Is Your Cooling Strategy Burning Your Profits? A Cost-Benefit Analysis of Vacuum vs. Conventional Cooling
As a bakery owner, you look at your balance sheet every month. You see the cost of ingredients, labor, and

Hybrid Cooling Solutions: Can Combining Vacuum and Traditional Methods Save Your Bakery?
Are you torn between the speed of new technology and the reliability of traditional freezing? Many bakery owners feel they

Blast Freezers vs Vacuum Coolers: Which Is the Right Choice for Your Bakery?
Are you tired of watching your freshly baked bread sit on racks for hours, taking up valuable space while you

Bakery Vacuum Cooling vs Spiral Cooling Systems: Which Is Better for Your Business?
Are you struggling with slow production lines because your bread takes forever to cool down? You are not alone. Cooling

Is a Vacuum Cooler the Missing Ingredient in Your Hotel or Restaurant Bakery?
Imagine your kitchen is in full swing, and the freshly baked bread is finally out of the oven, but it

Can One Cooling System Handle Your Entire Bakery Menu?
You juggle sourdough, delicate pastries, and frozen dough lines daily. Yet, one cooling mistake ruins the texture of your artisan

Is Your Frozen Dough Production Losing Quality Before It Hits the Freezer?
You watch your energy bills climb month after month while your blast freezers work overtime. You see production bottlenecks form

How Can Organic Bakeries Extend Shelf Life Without Preservatives?
You pour your heart into sourcing the finest organic flours and perfecting natural fermentation, only to watch your hard work

Can Vacuum Cooling Finally Fix the "Gummy" Texture in Gluten-Free Bread?
You watch your perfectly risen gluten-free loaves come out of the oven, only to see them sink or turn gummy
