How Does Playing with Pressure Help Cool Your Vegetables Super Fast?
Ever wondered if there’s a ‘shortcut’ to chilling vegetables without waiting hours for a cold room to do its job? The secret lies in understanding a fundamental relationship in physics – how pressure affects temperature.
By dramatically lowering air pressure, vacuum cooling exploits the direct link between pressure and the boiling point of water. This allows water on and in vegetables to boil (evaporate rapidly) at very low temperatures, quickly drawing heat from the produce and cooling it efficiently.

This might sound a bit technical, but it’s the core principle that makes vacuum cooling a game-changer for preserving vegetable freshness. Let’s break down this crucial relationship and see how it’s practically applied to get your veggies cool in record time.
What is the Basic Link Between Pressure and Temperature for Water?
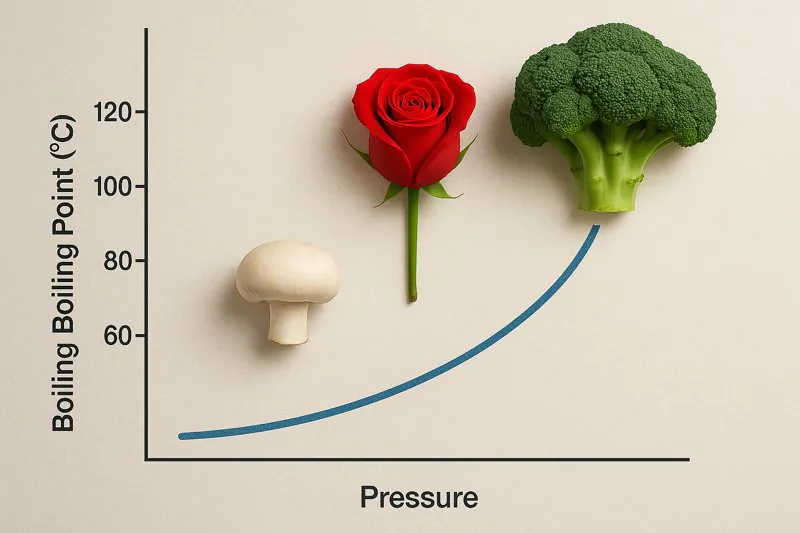
We all know water boils at 100°C (212°F) when we make tea, right? But is that temperature fixed? Not quite. That boiling point is true only under specific pressure conditions, something many people don’t realize impacts much more than just cooking.
The boiling point of water (the temperature at which it changes from liquid to gas) is directly dependent on the surrounding pressure. Higher pressure means a higher boiling point, and critically for vegetable cooling, lower pressure means a lower boiling point.

I often use the analogy of cooking at high altitudes to explain this to clients. If you’ve ever been in the mountains and tried to boil water, you’ll notice it boils at a temperature below 100°C. This is because the atmospheric pressure is lower at higher altitudes. There’s less "weight" of air pressing down on the water, so the water molecules find it easier to escape into the gaseous phase (steam) at a lower temperature.
This principle is fundamental:
- At Standard Atmospheric Pressure1 (Sea Level): Approximately 1013 millibars (mbar) or 1 atmosphere (atm). Water boils at 100°C (212°F).
- At Lower Pressures (e.g., High Altitude or in a Vacuum Chamber): Water molecules require less energy (and therefore a lower temperature) to overcome the reduced external pressure and transition into vapor.
- At Higher Pressures (e.g., Inside a Pressure Cooker2): Water boils at temperatures above 100°C. This is why pressure cookers cook food faster – the water gets hotter before it turns to steam.
Here’s a simple table showing this relationship:
| Pressure Condition | Approx. Pressure (mbar) | Approx. Water Boiling Point | Example Scenario |
|---|---|---|---|
| High (Pressure Cooker2) | ~2000 (2 atm) | ~121°C (250°F) | Faster cooking |
| Standard (Sea Level) | 1013 (1 atm) | 100°C (212°F) | Normal boiling |
| Low (High Mountain, ~5500m) | ~500 (0.5 atm) | ~81°C (178°F) | Slower cooking, water boils "cooler" |
| Very Low (Vacuum Cooler) | 6 – 30 mbar | 0°C – 25°C (32°F – 77°F) | Rapid evaporative cooling of vegetables |
Understanding this direct relationship is the first step to seeing how vacuum cooling works its magic on vegetables. It’s not about adding heat to boil water; it’s about reducing pressure so water boils at the existing temperature of the vegetables, or even lower.
How is This Pressure-Temperature Principle Used in Vacuum Coolers?
So we know lower pressure means a lower boiling point for water. How do we take this scientific fact and turn it into a practical way to chill tonnes of lettuce or broccoli quickly and efficiently? That’s where the engineering of a vacuum cooler comes in.
Vacuum coolers create a very low-pressure environment inside a sealed chamber. This forces the water on and within the vegetables to rapidly evaporate (boil) at the ambient temperature of the produce. This evaporation extracts heat, thereby cooling the vegetables.
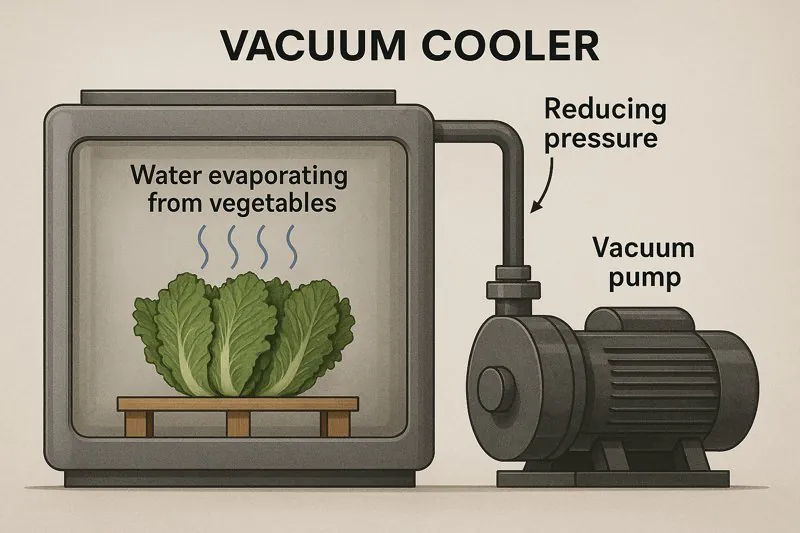
When I explain the process to someone like Carlos, who runs a large farm and needs rapid cooling for his produce, I emphasize how we’re manipulating the environment to benefit the vegetables. We’re not fighting against nature; we’re using its laws to our advantage.
Here’s how it’s applied step-by-step:
- Loading and Sealing: Vegetables are loaded into a strong, airtight chamber. The door is sealed to create an isolated environment.
- Evacuating Air (Reducing Pressure)3: Powerful vacuum pumps start removing air from the chamber. As air is pumped out, the pressure inside the chamber drops dramatically – from normal atmospheric pressure (around 1013 mbar) down to typically between 6 and 30 mbar.
- Reaching the "Flash Point": As the pressure falls, it eventually reaches a point where the boiling temperature of water matches the current temperature of the vegetables. For example, if the lettuce is at 25°C (77°F), water will start to boil vigorously when the pressure drops to around 31.7 mbar. This is often called the "flash point."
- Evaporative Cooling4: Once this low-pressure boiling begins, water from the surface and just below the surface of the vegetables rapidly turns into vapor. To do this, the water needs energy – the latent heat of vaporization. It draws this energy directly from the vegetables themselves.
- Temperature Drop5: As the vegetables lose heat energy to the evaporating water, their temperature quickly falls. For every 1% of moisture evaporated, the product temperature drops by about 6°C (11°F).
- Vapor Removal: The water vapor produced is continuously pumped out and condensed on refrigerated coils, maintaining the low pressure and allowing the cooling to continue until the desired vegetable temperature is reached.
This application means we can cool produce from, say, 30°C (86°F) down to 2°C (36°F) in typically 15-30 minutes. The cooling is highly uniform because the pressure drop affects all exposed surfaces of the vegetables equally, causing evaporation and thus cooling to occur all over, not just from one side.
| Stage | Pressure Level | Water Behavior on Vegetables | Result for Vegetables |
|---|---|---|---|
| Start (Ambient) | ~1013 mbar | Normal surface moisture | No cooling yet |
| Pressure Dropping | 1013 mbar -> ~30 mbar | Little change until flash point is near | Slight cooling from minor evaporation |
| At Flash Point / Evaporation | ~30 mbar to ~6 mbar | Vigorous boiling/evaporation at existing vegetable temperature | Rapid and uniform temperature drop |
| End of Cycle | ~6 mbar (example) | Evaporation slows as target temperature is reached | Vegetables cooled to target |
This elegant use of the pressure-temperature relationship makes vacuum cooling incredibly efficient for the right kinds of produce.
Why is Controlling Both Pressure and Temperature So Key for Vegetable Quality?
It’s not just about getting vegetables cold; it’s about getting them cold fast and gently to maintain their quality, extend shelf life, and ultimately satisfy customers like Norman, who are very discerning about quality. Simply dropping the pressure isn’t enough if not controlled properly.
Precisely controlling the rate of pressure drop and the final pressure level is crucial. This ensures rapid yet gentle cooling, preventing cell damage from ice formation (if cooled too low) and minimizing moisture loss, thus preserving the texture, crispness, and weight of the vegetables.

I always stress to my clients that our allcold systems are designed for this precise control. It’s the difference between a crude vacuum application and a sophisticated food preservation technology. For instance, if you pull the vacuum too deep too quickly on very delicate produce, or take it to a pressure where water would boil at a temperature below freezing, you could risk freezing injury on the product surface.
Here’s why careful control is paramount:
- Preventing Freezing6: The target is usually to cool vegetables to just above their freezing point (e.g., 1-4°C or 34-39°F). If the pressure is lowered so much that water boils at, say, -2°C (28°F), and the product temperature reaches that point, ice crystals can form within the plant tissues, causing damage and mushiness upon thawing. Our systems have controls to prevent this, stopping the cycle or adjusting pressure when the target temperature is reached.
- Minimizing Moisture Loss7: While some moisture loss (typically 1-3%) is inherent and necessary for evaporative cooling, excessive moisture loss can lead to wilting and weight reduction. The speed of vacuum cooling means the total time the product is losing moisture is very short compared to slower methods. Control systems help manage this. Some advanced vacuum coolers even have features to add a fine mist of water (a "hydro-vac" system) before or during the cycle for products that are prone to dehydration or don’t have enough free surface moisture.
- Ensuring Uniformity: While naturally more uniform than other methods, precise pressure management ensures that all vegetables in the batch, regardless of their position, experience similar cooling rates.
- Cycle Time Optimization8: Intelligent control systems monitor product temperature (often with wireless probes) and adjust the vacuum cycle to achieve the target temperature as quickly and efficiently as possible, without overcooling or wasting energy.
This sophisticated interplay between pressure and temperature management is what elevates vacuum cooling.
| Control Aspect | Importance for Vegetable Quality | How Achieved in Vacuum Cooler |
|---|---|---|
| Final Pressure Limit | Prevents water boiling at sub-freezing temps, avoiding ice damage | Pressure sensors, PLC control of vacuum pumps & vent valves |
| Rate of Pressure Drop | Gentle handling of delicate produce | Programmed vacuum pump operation, controlled venting |
| Product Temperature | Reaching optimal storage temp without overcooling or undercooling | Temperature probes (often wireless), feedback to control system |
| Moisture Management | Maintaining turgidity and saleable weight | Rapid cycle limits exposure; optional pre-wetting systems |
By mastering the application of the pressure-temperature relationship, we provide a superior cooling solution that directly benefits the quality and marketability of our clients’ produce.
Conclusion
The powerful link between pressure and water’s boiling temperature is the scientific cornerstone of vacuum cooling. By skillfully reducing pressure, we can make vegetables chill themselves from the inside out, quickly, uniformly, and efficiently, preserving their freshness for longer.
-
Understanding Standard Atmospheric Pressure is crucial for grasping how boiling points change under different conditions. Explore this link for detailed insights. ↩
-
Discover the science behind pressure cookers and how they utilize higher pressure to cook food more efficiently. This resource will enhance your cooking knowledge. ↩ ↩
-
Understanding this process is crucial for effective vacuum sealing, ensuring food preservation and quality. ↩
-
Exploring this concept helps grasp how temperature control is achieved during vacuum sealing, enhancing food safety. ↩
-
Learning about temperature drop is essential for optimizing vacuum sealing techniques and improving food storage. ↩
-
Explore this link to understand effective strategies for preventing freezing during vacuum cooling, ensuring product quality. ↩
-
Discover techniques to minimize moisture loss in vacuum cooling, which is crucial for maintaining the freshness of vegetables. ↩
-
Learn about advanced methods for optimizing cycle time in vacuum cooling, enhancing efficiency and energy savings. ↩

Mila
You May Also Like
How Do Lettuce Vacuum Coolers Actually Work: A Complete Technical Explanation?
You spend months growing the perfect lettuce, but field heat can turn your crisp harvest into wilted waste in hours.

What is the Best Lettuce Vacuum Cooler for Your Farm in 2026?
Are you watching your fresh lettuce wilt before it even reaches the supermarket shelves? You work hard to harvest, but

How Do You Handle the Peak Season Vegetable Rush?
The harvest season is here. Your fields are full of beautiful produce, but now you face the biggest challenge: a

Can You Vacuum Cool Vegetables After They Are Packaged?
You’ve just packed bags of beautiful, fresh-cut salad mix. But the product is still warm from processing and washing. This

How Do You Perfectly Cool Leafy Greens Without Damaging Them?
You’ve invested in a vacuum cooler to protect your leafy greens, but the results aren’t always perfect. Sometimes the lettuce

Will Your Vegetables Work in a Vacuum Cooler?
You’ve harvested a perfect crop, but the clock is ticking. Every minute of field heat is degrading the quality, reducing

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Are You Gambling with Your Export-Quality Vegetables?
You’ve grown a perfect crop, meeting every standard for size, color, and taste. Now comes the biggest challenge: shipping it

Is Your Cold Chain Broken Before It Even Starts?
Your company has invested millions in refrigerated trucks, state-of-the-art warehouses, and sophisticated inventory systems—a world-class cold chain. Yet, you’re still

Is Vacuum Cooling a Non-Negotiable Tool for Organic Growers?
As an organic producer, you’ve committed to a higher standard. Your customers pay a premium for vegetables that are not
