
How Does Vacuum Cooling Actually Chill Your Vegetables So Fast?
Ever wondered how vacuum coolers make vegetables cold in minutes without direct contact with ice or freezing air? It seems almost magical, but it’s all down to some very clever science.
Vacuum cooling chills vegetables by rapidly lowering the air pressure around them. This causes water on and within the produce to boil at a low temperature. This "boiling" (which is actually rapid evaporation) needs energy, and it takes this energy directly from the vegetables, cooling them down quickly and uniformly.

Now that you have the basic idea, it sounds pretty neat, right? But "boiling" to cool things? That might sound a bit strange. Let’s unpack the fascinating physics behind this process to see exactly how it works.
What Exactly is "Boiling" at Low Temperatures in Vacuum Cooling?
When you hear "boiling," you probably think of a pot of water on a stove at 100°C (212°F), right? So, how can boiling possibly cool vegetables down without cooking them? It’s a common point of confusion.
In vacuum cooling, "boiling" doesn’t mean high heat. It refers to the rapid phase change of water from liquid to gas (evaporation) that occurs at very low temperatures because the air pressure inside the vacuum chamber is dramatically reduced. It’s all about pressure, not heat input.

I often explain this to clients who are new to the technology. They’ll say, "Mila, are you sure it won’t cook my lettuce?" And I reassure them that it’s quite the opposite! The science is actually quite straightforward once you understand one key relationship: the link between pressure and the boiling point of water.
At sea level, with normal atmospheric pressure, water boils at 100°C (212°F). However, if you reduce the pressure, water will boil at a much lower temperature.
Think about cooking at high altitudes. If you’ve ever tried to boil an egg on a high mountain, you’ll know water boils at a temperature lower than 100°C because the atmospheric pressure is lower up there. A vacuum chamber takes this principle to an extreme. By pumping out most of the air, we create a very low-pressure environment.
Inside the vacuum chamber, once the pressure drops sufficiently, the water on the surface of the vegetables and just beneath the surface can "boil" – or more accurately, rapidly evaporate – even if the vegetables are at room temperature, say 25°C (77°F), or even cooler. The vegetables themselves don’t get hot; the water just changes state very, very quickly.
Here’s a simplified look at how pressure affects water1‘s boiling point:
| Pressure | Approximate Boiling Point of Water | Environment Example |
|---|---|---|
| 1 atm (1013 mbar) – Normal Sea Level | 100°C (212°F) | Standard kitchen stove |
| 0.5 atm (approx. 500 mbar) | ~81°C (~178°F) | High mountain (e.g., Mt. Whitney summit) |
| 0.03 atm (approx. 30 mbar) | ~25°C (~77°F) | Typical in vacuum cooling2 |
| 0.006 atm (approx. 6 mbar) – Triple Point | ~0.01°C (~32°F) | Achievable in vacuum coolers for near-freezing temps |
So, the "boiling" in vacuum cooling is a controlled, low-temperature phenomenon entirely driven by reduced pressure, not by adding heat. This is key to its effectiveness.
How Does This Evaporation Actually Cool the Vegetables? (The Physics of Evaporative Cooling)
Okay, so we’ve established that water can "boil" or evaporate very quickly at low temperatures under vacuum. But how does the act of water turning into vapor actually remove heat from the broccoli, lettuce, or mushrooms? It’s not just about the water disappearing.
Evaporation is an endothermic process, meaning it requires an input of energy (heat) to occur. This energy is called the "latent heat of vaporization." In a vacuum cooler, this heat energy is drawn directly from the vegetables themselves, thus lowering their temperature rapidly.
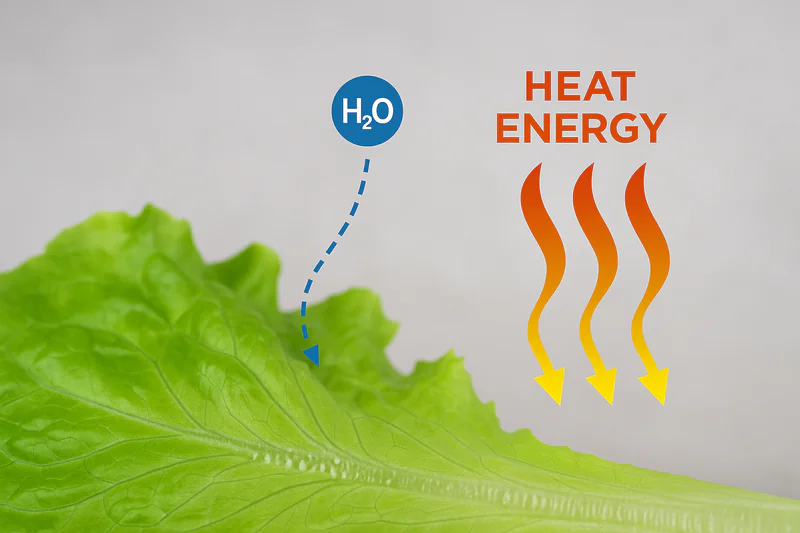
This is the real magic behind vacuum cooling, and it’s a fundamental principle of physics. I find that using an everyday example helps make this clear. Think about how you feel when you step out of a swimming pool on a warm day. Even if the air is warm, you often feel chilly as the water evaporates from your skin. That’s evaporative cooling in action! The water on your skin needs energy to turn into vapor, and it takes that energy from your body, making you feel cooler.
The same thing happens to vegetables in a vacuum cooler, but much more intensely.
- Latent Heat of Vaporization3: This is the specific amount of heat energy absorbed by a substance when it changes from a liquid to a gas, without changing its temperature. For water, this is a significant amount of energy (about 2,260 kilojoules per kilogram, or 970 BTU per pound).
- Energy Source4: To evaporate, the water molecules on and within the vegetables must gain this latent heat energy. In the isolated environment of the vacuum chamber, the most readily available source of this energy is the vegetables themselves.
- Cooling Effect5: As the water molecules absorb heat from the vegetable to transform into vapor, they carry this heat away, effectively cooling the vegetable.
A crucial point is that only a small amount of the vegetable’s total moisture needs to evaporate to achieve significant cooling. Typically, for every 1% of its weight lost as water vapor, the product’s temperature will drop by approximately 6°C (or about 11°F). So, to cool vegetables from 25°C (77°F) down to 1°C (34°F), you’d need a temperature drop of 24°C. This would require roughly a 4% moisture loss (24°C / 6°C per % = 4%). In practice, it’s often even less, around 1-3%, because the process is so efficient. This small moisture loss is generally acceptable and doesn’t harm the quality of most produce; in fact, it can sometimes even firm up leafy greens. The water comes from free surface moisture and some moisture from within the plant tissues near the surface.
Here’s a simple illustration:
| Product | Initial Temp. | Target Temp. | Temp. Drop Needed | Approx. Moisture Evaporated |
|---|---|---|---|---|
| Lettuce | 28°C (82°F) | 2°C (36°F) | 26°C (46°F) | ~4.3% |
| Broccoli | 22°C (72°F) | 1°C (34°F) | 21°C (38°F) | ~3.5% |
| Mushrooms | 18°C (64°F) | 2°C (36°F) | 16°C (28°F) | ~2.7% |
This powerful cooling mechanism is why vacuum cooling is so incredibly fast and uniform. The cooling happens wherever water can evaporate, meaning it cools from the surface and even slightly within the product simultaneously.
What Happens to the Water Vapor Inside the Vacuum Chamber?
So, if water is rapidly "boiling" off the surface of all those vegetables, creating water vapor, where does it all go? Does the vacuum chamber just fill up with steam? That wouldn’t be very effective for long.
The water vapor generated from the vegetables is continuously drawn out of the main chamber by the vacuum pump. It then passes over very cold refrigeration coils (the condenser or evaporator), where it condenses back into liquid water or even turns directly into ice. This removes the vapor and its heat from the system.

This part of the system is critical for maintaining the vacuum and ensuring continuous cooling. I explain to clients like Sophia, who is very detail-oriented and concerned with hygiene and efficiency, that this controlled removal of water vapor is key to the consistent performance and cleanliness of the process.
Here’s how the components work together:
- Vacuum Pump(s)6: Their primary job is to evacuate air from the sealed chamber to reach the low pressures needed for low-temperature boiling. But just as importantly, they continuously pump out the non-condensable gases and the water vapor that is constantly being produced by the evaporating vegetables.
- Condenser (Refrigeration Coils / Evaporator)7: This is the coldest part of the system inside the vacuum vessel (or connected to it). It consists of coils chilled by a refrigeration system, much like the evaporator coils in a fridge or air conditioner. As the warm, moist vapor drawn from the vegetables passes over these extremely cold coils, it rapidly cools down and condenses – changing from a gas back into a liquid (water) or solid (ice).
- Maintaining Low Pressure: By condensing the vapor, we remove it from the gaseous phase. If the vapor were allowed to accumulate, the pressure inside the chamber would rise, stopping the low-temperature boiling.
- Removing Latent Heat8: When the water vapor condenses, it releases the latent heat of vaporization it absorbed from the vegetables. This heat is now transferred to the refrigerant in the condenser coils and is ultimately expelled outside the vacuum system by the refrigeration unit’s own condenser (usually air-cooled, outside the vacuum chamber).
Essentially, the vacuum cooling system acts as a highly efficient heat pump. It uses the principle of evaporative cooling to extract heat from the vegetables and then uses a refrigeration system to collect and eject that heat. Some systems, often called "hydro-vacuum coolers" or systems with "ice banks," build up a large reserve of ice on the coils before the cooling cycle starts. This ice provides a massive cold surface area for very rapid condensation of the water vapor.
| Component | Role in Vapor Management | Consequence if Not Working Properly |
|---|---|---|
| Vacuum Pump | Removes air & water vapor from chamber | Pressure rises, evaporation slows/stops |
| Condenser Coils | Provides cold surface for vapor to condense on | Vapor accumulates, pressure rises |
| Refrigeration System | Cools the condenser coils, removes collected heat | Coils warm up, condensation ineffective |
This efficient capture and removal of water vapor ensures that the vacuum conditions are maintained, allowing the evaporative cooling process to continue rapidly until the vegetables reach their target temperature.
Conclusion
In essence, vacuum cooling brilliantly uses the physics of low-pressure evaporation. By making water "boil" at low temperatures, it forces the vegetables to give up their heat quickly and uniformly, locking in freshness in a way that’s hard to beat.
-
Learning about the relationship between pressure and water’s properties can deepen your understanding of physical science and its real-world applications. ↩
-
Exploring vacuum cooling will reveal its innovative applications in food preservation and cooking, showcasing modern culinary techniques. ↩
-
Understanding the Latent Heat of Vaporization is essential for grasping how cooling processes work, especially in food preservation. ↩
-
Learning about the Energy Source in vacuum cooling can help optimize energy use in food processing and preservation. ↩
-
Exploring the Cooling Effect can provide insights into efficient cooling methods that enhance food quality and shelf life. ↩
-
Understanding vacuum pumps is crucial for optimizing food preservation techniques. Explore this link to enhance your knowledge. ↩
-
Learn about the role of refrigeration coils in food preservation to improve your understanding of effective cooling methods. ↩
-
Discover the significance of latent heat in food processing, which is essential for effective preservation techniques. ↩

Mila
You May Also Like

How Do You Handle the Peak Season Vegetable Rush?
The harvest season is here. Your fields are full of beautiful produce, but now you face the biggest challenge: a

Can You Vacuum Cool Vegetables After They Are Packaged?
You’ve just packed bags of beautiful, fresh-cut salad mix. But the product is still warm from processing and washing. This

How Do You Perfectly Cool Leafy Greens Without Damaging Them?
You’ve invested in a vacuum cooler to protect your leafy greens, but the results aren’t always perfect. Sometimes the lettuce

Will Your Vegetables Work in a Vacuum Cooler?
You’ve harvested a perfect crop, but the clock is ticking. Every minute of field heat is degrading the quality, reducing

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Are You Gambling with Your Export-Quality Vegetables?
You’ve grown a perfect crop, meeting every standard for size, color, and taste. Now comes the biggest challenge: shipping it

Is Your Cold Chain Broken Before It Even Starts?
Your company has invested millions in refrigerated trucks, state-of-the-art warehouses, and sophisticated inventory systems—a world-class cold chain. Yet, you’re still

Is Vacuum Cooling a Non-Negotiable Tool for Organic Growers?
As an organic producer, you’ve committed to a higher standard. Your customers pay a premium for vegetables that are not

Can Small Farms Actually Afford a Vacuum Cooler?
You’ve poured your heart into your farm, producing the highest quality vegetables. But as soon as they’re picked, the summer
How Do You Customize a Vacuum Cooling Cycle for Different Vegetables?
You’ve invested in a powerful vacuum cooler, expecting it to be a simple "set it and forget it" solution. But
