
Why is Evaporation the Secret Engine of Vacuum Cooling?
You see a pallet of hot, freshly baked bread or warm, just-picked spinach. You know that slow cooling means lower quality and lost profits. You need a way to pull that heat out fast, but traditional methods are just too inefficient.
Evaporation is the engine because when water turns to gas, it absorbs huge amounts of heat from its surroundings. Vacuum cooling creates the perfect low-pressure conditions for this to happen with incredible speed, cooling the product from the inside out.
This isn’t some new-age trick; it’s the same principle that cools you down after a swim. But when we harness this natural process inside a high-tech chamber, it becomes the most powerful cooling method for many food products. Let’s explore exactly how this mechanism works and why it’s so effective.
Why Do You Feel Cold After a Swim?
We’ve all experienced it. You step out of the pool on a warm day, and a slight breeze makes you shiver. You’re not cold because the air is cold; you’re cold because the water on your skin is actively pulling heat from your body.
You feel cold because the evaporating water requires energy to change from a liquid to a gas. It takes this energy directly from the nearest source—your skin—leaving the surface cooler.

Let’s dive deeper into the physics, but keep it simple. Water is made of tiny molecules. In liquid form, these molecules are close together and moving around. Some molecules move faster than others. The very fastest-moving molecules, those with the most energy, can break free from the surface and escape into the air as a gas (water vapor). This process is called evaporation. When these high-energy molecules leave, the average energy of the remaining molecules drops. Since heat is just a measure of this energy, the water left behind is cooler. Your skin is in direct contact with this now-cooler water, and the process continues, with the evaporating water constantly drawing heat away from your body. This is a perfect, everyday example of the evaporative cooling mechanism. In my business, we’ve simply created a machine that takes this natural phenomenon and puts it on an industrial scale. We don’t change the physics; we just create the ideal environment for it to happen with maximum efficiency.
How Does Low Pressure Supercharge Evaporation?
The cooling effect you feel after a swim is gentle and slow. A vacuum cooler, however, can chill a ton of mushrooms in 20 minutes. What’s the difference? The secret ingredient is the vacuum itself.
Air pressure constantly pushes down on the surface of water, making it difficult for molecules to escape. By removing this air pressure in a vacuum, we make it incredibly easy for water to evaporate, supercharging the cooling effect.
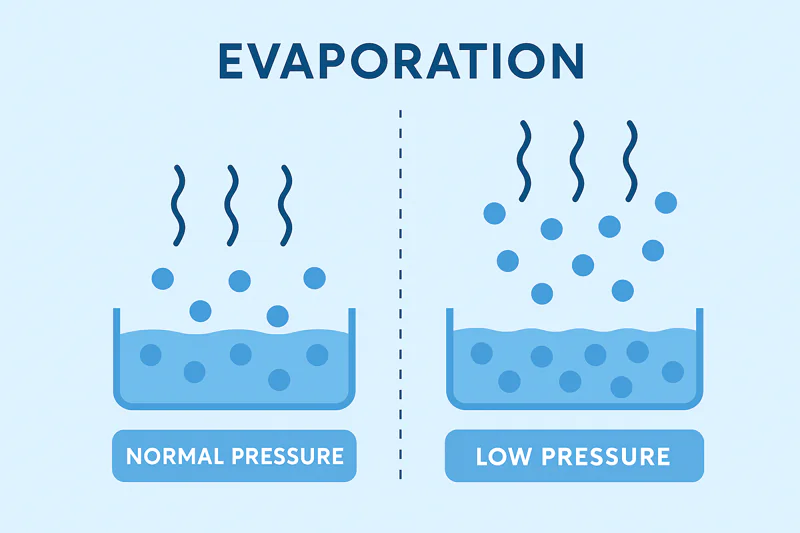
I often use this analogy with my clients: imagine trying to leave a very crowded room. It’s difficult and slow because you have to push past so many people. Now imagine leaving a nearly empty room. You can exit almost instantly. Air pressure is like that crowd. At normal atmospheric pressure, water molecules have to fight their way through a "crowd" of air molecules to evaporate. Inside a vacuum chamber1, we pump out almost all the air. We create that "empty room." With no air pressure holding them back, water molecules can escape with very little energy. This has a profound effect: it dramatically lowers the boiling point of water2. We’re not heating the water to boil it; we are creating an environment where it boils at a very low temperature. For a technical decision-maker like Carlos, seeing these numbers makes it click.
| Environment | Atmospheric Pressure | Water Boiling Point |
|---|---|---|
| Normal (Sea Level) | 101.3 kPa | 100°C (212°F) |
| Mount Everest Peak | ~33.7 kPa | ~70°C (158°F) |
| Allcold Vacuum Cooler | 0.6 kPa | ~1°C (34°F) |
This is the key. We can make water "boil" at a temperature that is safe and even beneficial for fresh produce, achieving rapid cooling without any risk of heat damage.
Where Does the Heat Energy Actually Go?
When a product cools, its heat energy has to go somewhere. In a standard freezer, it slowly leaks into the cold air. But in vacuum cooling, the heat is removed much more directly and forcefully.
The heat is captured and carried away by the water vapor itself. This process, called "latent heat of vaporization," is an incredibly efficient way to transfer energy. The evaporating water acts like a sponge, soaking up heat and removing it.
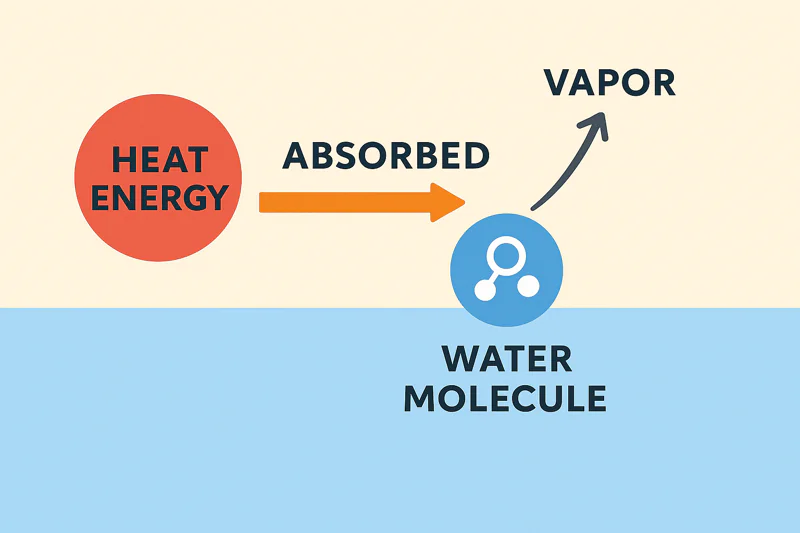
Let’s get into what "latent heat" really means. It’s the massive amount of hidden energy required for a substance to change its state (e.g., from liquid to gas) without changing its temperature. To turn one gram of liquid water into one gram of water vapor, it must absorb about 540 calories of heat energy. This is a huge number! To put it in perspective, it only takes 1 calorie to raise the temperature of that same gram of water by 1°C. This means the phase change from liquid to gas is a tremendously powerful energy sponge3. When I work with a client like Sophia from a central kitchen, this is the concept that excites her. She needs to cool thousands of kilograms of cooked rice or stew from 90°C to below 10°C. That’s a massive heat load. The latent heat of vaporization4 is the only mechanism that can pull that much heat out, from the very core of the product, in the short time her production schedule allows. The water evaporating from the food literally carries the heat energy with it, which is then frozen onto the ice-catcher coils inside my Allcold machine.
Is There a Trade-Off to Using Evaporation for Cooling?
When I explain this process, smart buyers like Norman always ask the right question: "This sounds great, but what’s the catch?" They are right to be skeptical. In business, there are always trade-offs, and transparency is key to building trust.
Yes, there is one main trade-off: a small and predictable weight loss. Because cooling is caused by water turning to vapor and leaving the product, that water has mass. Therefore, the product’s final weight is slightly reduced.
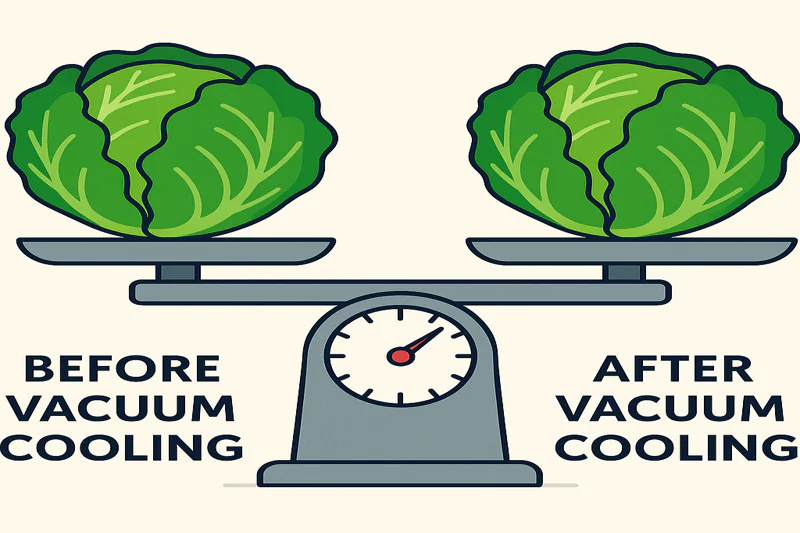
This is the most critical point to understand from a commercial perspective. Luckily, this weight loss is not random; it’s directly proportional to the amount of cooling performed. The rule of thumb I give all my clients is simple and reliable: you will see approximately 1% of weight loss for every 5°C (about 10°F) of cooling you achieve. So, if you cool a pallet of lettuce from 25°C down to 5°C (a 20°C drop), you can expect a weight loss of about 4%. For a buyer like Norman, this is crucial information for calculating yield and cost. But here’s how we frame it: what is the alternative? Without this rapid cooling, spoilage, wilting, and moisture loss during storage and transport could easily lead to a 15-30% loss. A predictable 4% loss is a fantastic insurance policy against a much larger, unpredictable one. Furthermore, what is lost is only pure water, which can sometimes even slightly concentrate the product’s flavor. It’s a calculated trade-off that massively extends shelf life, locks in freshness, and ultimately protects your investment.
Evaporative Weight Loss: A Balanced View
| The "Con" (The Cost) | The "Pro" (The Benefit) |
|---|---|
| A predictable 1% weight loss5 per 5°C of cooling. | Prevents 15-30% of spoilage and quality loss. |
| Only pure water weight is removed. | Greatly extends product shelf life6 and saleable window. |
| Requires the product to have available moisture. | Locks in freshness, color, and texture. |
| This is a cost that can be calculated and managed. | Drastically reduces product waste and financial losses. |
Conclusion
The evaporative cooling mechanism is the heart of what we do. By harnessing this powerful, natural process, we provide not just cooling, but a guarantee of quality, speed, and extended shelf life for your valuable products.
-
Exploring how vacuum chambers operate can provide insights into their applications in various scientific and industrial fields. ↩
-
Understanding the boiling point in a vacuum is crucial for applications in food preservation and cooling techniques. ↩
-
Exploring this term will enhance your grasp of energy transfer in phase changes, vital for various scientific and industrial applications. ↩
-
Understanding this concept is crucial for efficient cooling processes in food production, especially in large-scale operations. ↩
-
Understanding weight loss in food preservation can help you appreciate its role in extending shelf life and reducing waste. ↩
-
Exploring shelf life insights can enhance your knowledge of food quality management and waste reduction strategies. ↩

Mila
You May Also Like

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Why Do Vacuum Cooler Energy Costs Vary So Much?
You’re calculating the return on investment for a new vacuum cooler, but there’s a huge unknown: the electricity bill. You
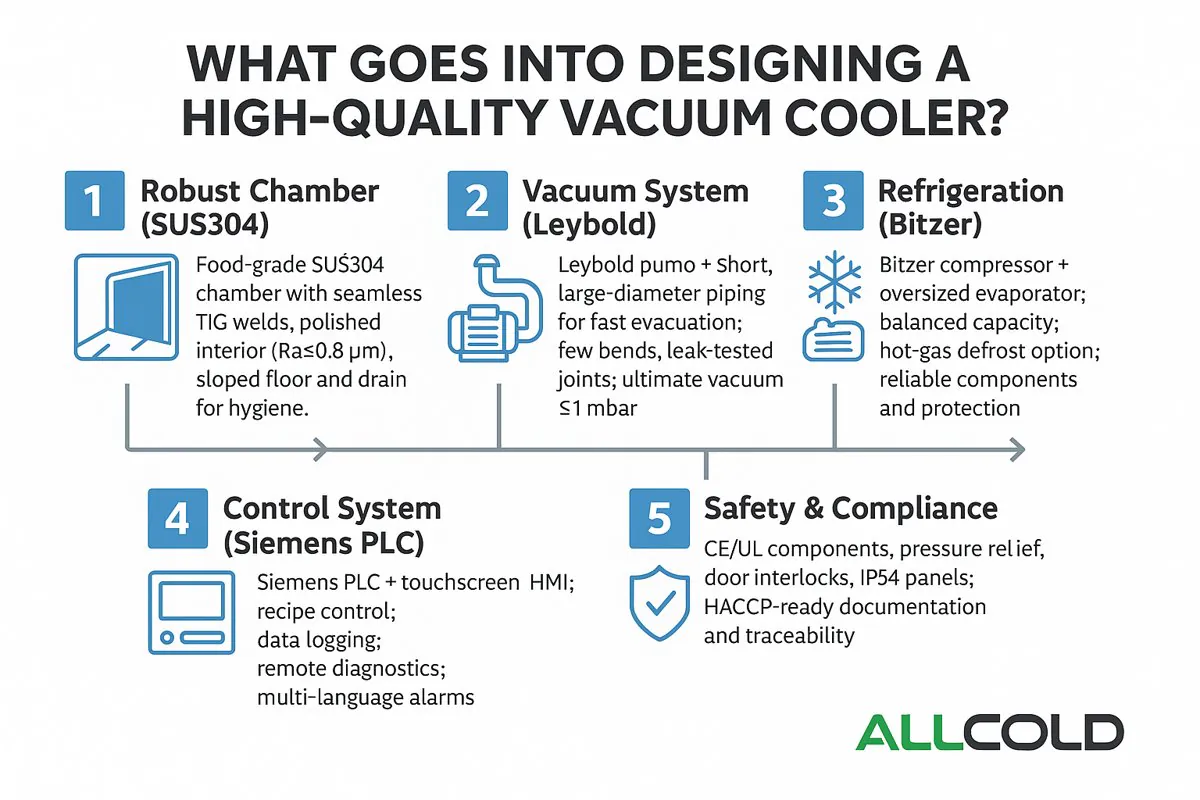
What Goes Into Designing a High-Quality Vacuum Cooler?
At first glance, a vacuum cooler seems simple: it is a steel box that makes vegetables cold. But this simplicity

What Are the Most Important Features in a Vacuum Cooler?
You’re comparing quotes from different suppliers, and the specification sheets all start to look the same. They all list a

Where Can You Find Reliable Vegetable Vacuum Cooler Suppliers?
You’ve made the decision to invest in a vacuum cooler, but now you face an even bigger challenge: finding a
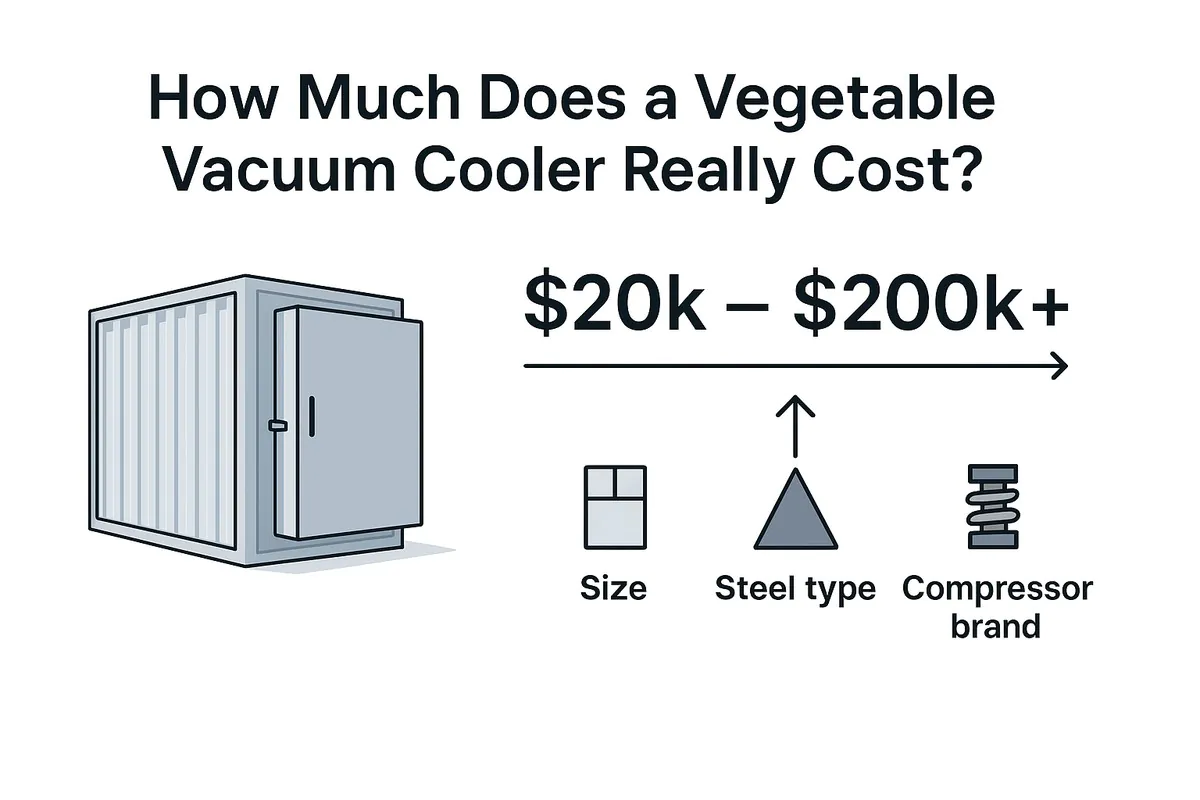
How Much Does a Vegetable Vacuum Cooler Really Cost?
You know you need a vacuum cooler to improve your product quality, but the price is a huge question mark.

Industrial vs. Commercial Vacuum Coolers: Which Should You Choose?
Your business is growing, and you know that rapid post-harvest cooling is the key to quality and profit. But as

What's the Real Difference Between a High-Quality and a Low-Price Vacuum Cooler?
You are looking for a vacuum cooler, and you see a huge range of prices. One supplier quotes a price

How Do You Choose the Best Vegetable Vacuum Cooler in 2024?
Choosing a vacuum cooler is one of the biggest investments you’ll make in your farm or food business. The market

What Can You Learn from Farms That Mastered Vacuum Cooling?
You see the challenges in your own operation every day: the race against field heat, the constant worry about shelf
