
How Does Heat Actually Move During Vacuum Cooling?
You have a big challenge: a mountain of product, full of heat. You know that getting this heat out quickly and evenly is the key to quality and profit. But heat is stubborn; it doesn’t like to move fast. So how can a vacuum chamber pull it out in minutes?
Heat transfer in vacuum cooling is driven by rapid evaporation. When water boils at a low temperature, it absorbs heat energy (a process called a phase change) and carries it away as vapor. This happens all over the product’s surface at once.
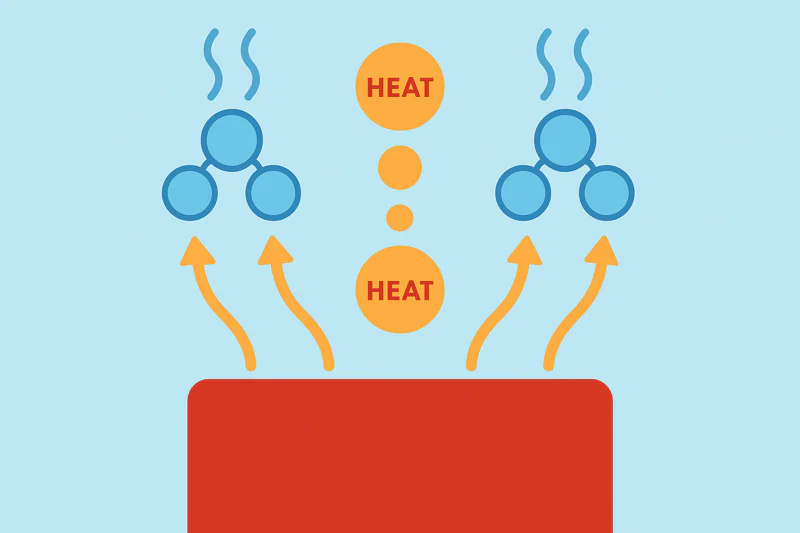
Unlike a traditional freezer that slowly pulls heat through the air, vacuum cooling uses the product’s own moisture as the vehicle for heat removal. It’s an incredibly direct and powerful method. To really grasp why this is a game-changer, we need to compare it to the old way and explore how heat moves both from and within your product.
How is this different from a normal cold room?
When you place warm products in a traditional cold room or blast chiller, you are starting a very slow battle. You are trying to cool a solid or liquid product using cold air. This is a process called convection and conduction, and it is famously inefficient.
A cold room cools from the outside-in, relying on slow air convection. A vacuum cooler cools from the inside-out, powered by rapid phase change evaporation. The difference in speed and uniformity is immense.

Let’s dive deeper into these two very different dynamics. In a standard cold room, a refrigeration unit cools the air. Then, large fans blow this cold air over your pallets. The heat must first transfer from the surface of your product into the air (convection). This air then circulates back to the refrigeration unit to get re-cooled. Meanwhile, the heat from the center of your product must slowly travel through the product itself to reach the surface (conduction). This "outside-in" process is slow for several reasons. Air is a poor conductor of heat. Also, the process creates a huge temperature difference between the outside and the inside of the product, which can cause damage like freezer burn or surface dehydration. I once had a client, a large-scale baker, who struggled with this exact problem. His bread would be cold on the outside but still warm and steaming in the middle, creating condensation and spoilage issues. Vacuum cooling flips this entire dynamic. We are not using air to move the heat. We are creating a low-pressure environment where the product’s own water turns into vapor. This phase change is an incredibly efficient heat transfer mechanism, thousands of times more effective than air convection. Heat is removed instantly from every surface where water is present, leading to a much faster and more uniform result.
Heat Transfer Method Comparison
| Feature | Traditional Cold Room1 | Allcold Vacuum Cooler2 |
|---|---|---|
| Primary Mechanism | Convection & Conduction (via air) | Phase Change (Evaporation) |
| Direction of Cooling | Outside-in | Inside-out / Uniform |
| Speed | Very Slow (Hours) | Very Fast (Minutes) |
| Uniformity | Poor (Hot and cold spots) | Excellent |
| Risk | Surface freezing, dehydration, slow cooling | Minimal, predictable moisture loss |
How does heat get from the center to the surface?
This is a brilliant question that gets to the core of the science. If the evaporation is happening on the surface, how does the heat deep inside a loaf of bread or a dense head of cabbage get out? It doesn’t magically teleport. It moves through conduction, but in a very clever way.
The rapid evaporation aggressively cools the product’s surface. This creates a temperature difference between the cool surface and the warm core. Heat naturally flows from hot to cold, so the core’s heat moves towards the surface to be removed.
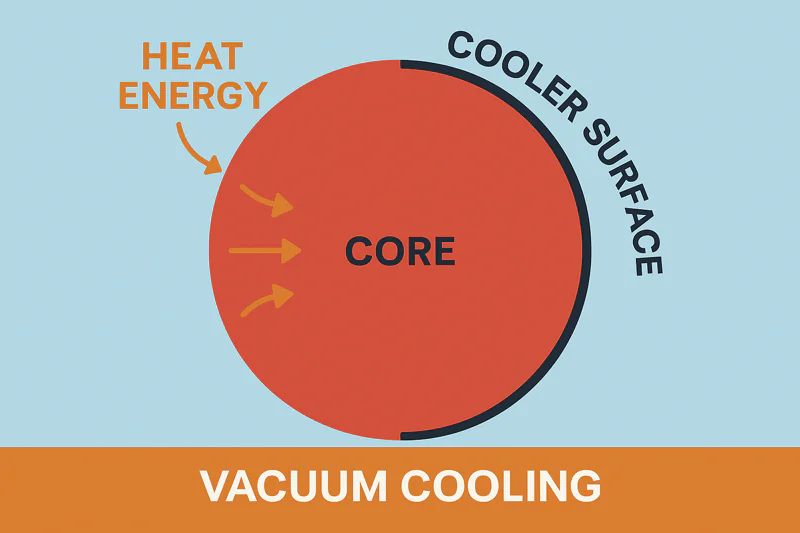
This is one of the most elegant aspects of the process. Let’s break it down. Imagine a head of cabbage at 25°C. When we start the vacuum cycle, the water on the outer leaves begins to boil at, say, 5°C. Instantly, those outer leaves are cooled down. Now we have a situation: the surface is near 5°C, but the core is still at 25°C. Physics dictates that heat energy must flow from a warmer area to a cooler area. So, the heat from the core begins to travel outwards towards the now-cold surface. As this heat reaches the surface, it provides more energy for the evaporation process, fueling the removal of even more heat. It creates a self-perpetuating cycle. The evaporation cools the surface, which causes heat to flow from the core, which then fuels more evaporation. This is fundamentally different from a cold room. In a cold room, the heat flow is sluggish because the temperature difference might be small and air is a poor heat acceptor. In a vacuum cooler3, the phase change is so powerful and happens so quickly that it creates a very steep "thermal gradient4," forcing the internal heat to move much, much faster than it normally would. This is why the process works so well even on relatively dense products. It’s an active, aggressive heat extraction process.
Why is the cooling effect so uniform and deep?
The number one enemy for food producers is a lack of uniformity. Hot spots in a pallet of produce or a batch of cooked stew lead directly to spoilage and waste. The biggest advantage of vacuum cooling is its incredible uniformity.
Because the vacuum is created throughout the entire chamber at once, the pressure drops equally everywhere. This means evaporation and cooling start simultaneously on all surfaces of the product, from top to bottom.
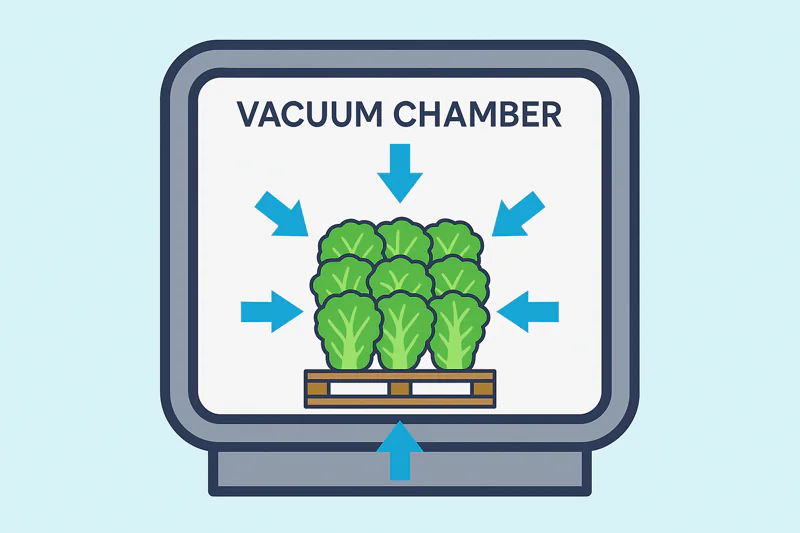
Think about how a blast chiller5 works. It has fans on one side. The boxes closest to those fans get blasted with cold air, while the boxes in the middle or on the far side are shielded. This creates a huge temperature difference across the pallet. I speak with farm owners like Carlos who have to deal with this all the time; the outside of the pallet is cold, but the center is still warm from the field heat. This is a recipe for disaster. Vacuum cooling solves this problem completely. The pressure in my Allcold vacuum chamber is the same in every single cubic centimeter. It doesn’t matter if a head of lettuce is at the top corner or buried in the very center of the pallet. The low pressure reaches it just the same. This means the cold boiling process begins on every leaf of every head of lettuce at the exact same moment. You have millions of tiny cooling engines firing at once, all over the pallet. This, combined with the rapid internal conduction we just discussed, results in a pallet that is uniformly cooled to within one or two degrees from corner to corner, top to bottom. For a central kitchen manager like Sophia, this is not just a benefit; it’s a requirement for food safety and quality control.
What is the ultimate destination of the heat?
So the heat moves from the core of your product to the surface, where it’s absorbed by evaporating water. But where does that heat-filled water vapor go? It doesn’t just stay in the chamber. It is actively captured and removed.
The heat, now stored in the water vapor, is drawn to the coldest point in the chamber: the refrigeration coils, also known as the ice-catcher. The vapor freezes onto these coils, trapping both the water and the heat it carries.

This is the final piece of the heat transfer puzzle and a common point of confusion. People see the large refrigeration unit on my machines and assume it’s what cools the product. It’s not! The refrigeration unit’s only job is to make the "ice-catcher6" coils extremely cold (around -20°C or -4°F). This super-cold surface acts like a magnet for the water vapor. As soon as the heat-laden vapor touches these coils, it instantly desublimates7—it turns directly from a gas into a solid (ice) without passing through a liquid stage. This is a critical step for two reasons. First, it permanently removes the heat from the environment. The heat that was in your lettuce is now locked away in the ice on those coils. Second, by removing the water vapor from the chamber, it helps the vacuum pump maintain the extremely low pressure needed to keep the process running. At the end of the cooling cycles, you simply run a "defrost" cycle, which melts this ice, and the water (which originally came from your product) drains away. It’s a complete, closed-loop system for capturing and disposing of unwanted heat.
Conclusion
The dynamics of heat transfer in vacuum cooling are a beautiful example of applied physics. It’s a system that uses evaporation, conduction, and phase change in a perfect symphony to achieve incredibly fast, uniform, and deep cooling results.
-
Explore the benefits of Traditional Cold Rooms to understand their role in food preservation and storage. ↩
-
Learn about the innovative technology behind Allcold Vacuum Coolers and their efficiency in rapid cooling. ↩
-
Explore this link to understand the mechanics of vacuum coolers and their efficiency in heat extraction. ↩
-
Learn about thermal gradients to grasp their significance in processes like vacuum cooling and heat transfer. ↩
-
Learn about blast chillers to see their limitations and why vacuum cooling is a superior alternative for consistent cooling. ↩
-
Understanding the role of the ice-catcher can enhance your knowledge of refrigeration technology and its efficiency. ↩
-
Exploring desublimation will deepen your grasp of phase changes in materials, crucial for various scientific applications. ↩

Mila
You May Also Like

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Why Do Vacuum Cooler Energy Costs Vary So Much?
You’re calculating the return on investment for a new vacuum cooler, but there’s a huge unknown: the electricity bill. You
What Goes Into Designing a High-Quality Vacuum Cooler?
At first glance, a vacuum cooler seems simple: it is a steel box that makes vegetables cold. But this simplicity

What Are the Most Important Features in a Vacuum Cooler?
You’re comparing quotes from different suppliers, and the specification sheets all start to look the same. They all list a

Where Can You Find Reliable Vegetable Vacuum Cooler Suppliers?
You’ve made the decision to invest in a vacuum cooler, but now you face an even bigger challenge: finding a
How Much Does a Vegetable Vacuum Cooler Really Cost?
You know you need a vacuum cooler to improve your product quality, but the price is a huge question mark.

Industrial vs. Commercial Vacuum Coolers: Which Should You Choose?
Your business is growing, and you know that rapid post-harvest cooling is the key to quality and profit. But as

What's the Real Difference Between a High-Quality and a Low-Price Vacuum Cooler?
You are looking for a vacuum cooler, and you see a huge range of prices. One supplier quotes a price

How Do You Choose the Best Vegetable Vacuum Cooler in 2024?
Choosing a vacuum cooler is one of the biggest investments you’ll make in your farm or food business. The market

What Can You Learn from Farms That Mastered Vacuum Cooling?
You see the challenges in your own operation every day: the race against field heat, the constant worry about shelf
