
How Does Lowering Pressure Make Things Cold?
You look at the control panel of a vacuum cooler, seeing a pressure gauge dropping while a temperature gauge follows it down. The link is obvious but feels like complex physics. You wonder how one number falling can magically cause another one to do the same.
This is not magic; it’s a direct physical law. Lowering pressure makes it easier for water to boil. This means it can boil at a much colder temperature. This cold boiling, or rapid evaporation, is what pulls the heat out of your product.
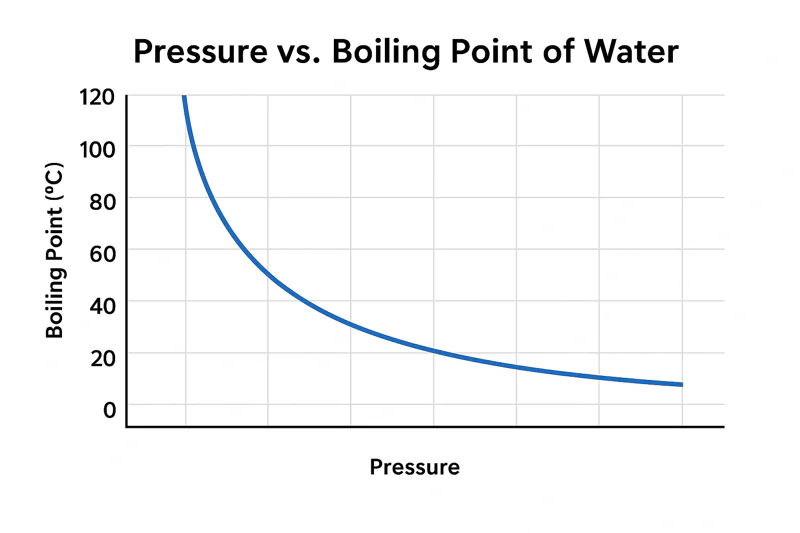
This core relationship is the engine of vacuum cooling. It’s more reliable than a car engine and as predictable as gravity. For a professional buyer like Norman or a hands-on farm owner like Carlos, truly understanding this link is the key to unlocking maximum efficiency and quality. Let’s explore exactly how this scientific principle works inside my Allcold machines.
Why Isn’t Water’s Boiling Point Always 100°C (212°F)?
We all learn in school that water boils at 100°C. This single fact is so ingrained in us that it makes vacuum cooling seem impossible. How can we make water boil on a lettuce leaf without cooking it? The answer is that the 100°C rule has a hidden condition.
The boiling point of water is not a fixed universal constant. It is entirely dependent on the atmospheric pressure pushing down on it. Change the pressure, and you change the boiling point.
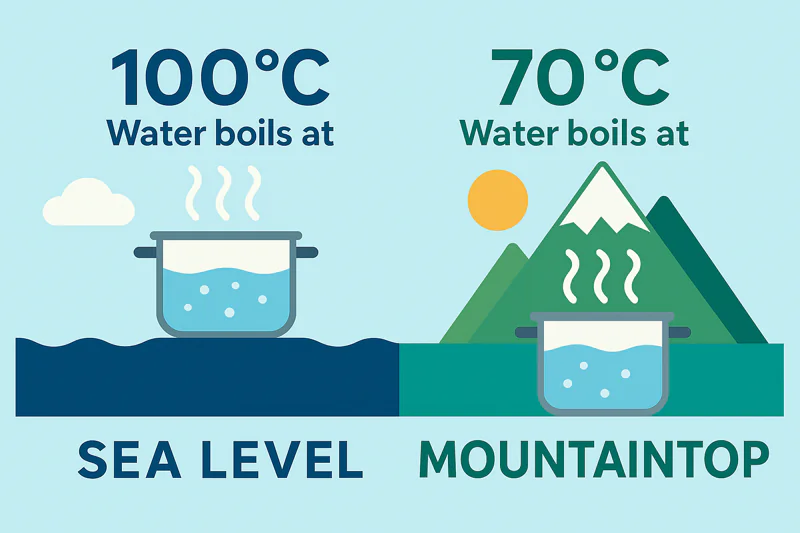
Think of atmospheric pressure1 as a physical weight or a lid pressing down on the surface of all water. For a water molecule to escape and turn into steam (boil), it needs enough energy to push that lid off. At sea level, where the pressure is high, it needs a lot of energy, which corresponds to a temperature of 100°C. Now, imagine you are on top of Mount Everest. The column of air above you is much shorter, so the pressure, or "weight," is much lower. With less weight pushing down, the water molecules need less energy to escape. On Everest, water boils at only about 70°C (158°F). We take this principle to the extreme. Inside one of my vacuum coolers2, we use a powerful pump to remove over 99% of the air. This reduces the pressure to an incredibly low level. With almost no "lid" holding the water molecules back, they can escape with very little energy. This means they can boil at just 2°C or 3°C (around 36°F). This is the secret. We are not adding heat; we are removing pressure to enable a safe, cold boiling process.
What is the "Pressure-Temperature Curve" in Simple Terms?
Engineers and scientists call this predictable link the "pressure-temperature saturation curve." That sounds technical and intimidating, but the concept is very straightforward. It’s simply the map that we follow.
The pressure-temperature curve is a rulebook that tells us exactly what pressure we need to create to make water boil at a specific target temperature. It is the precise "recipe" our machine’s computer uses for every cooling cycle.
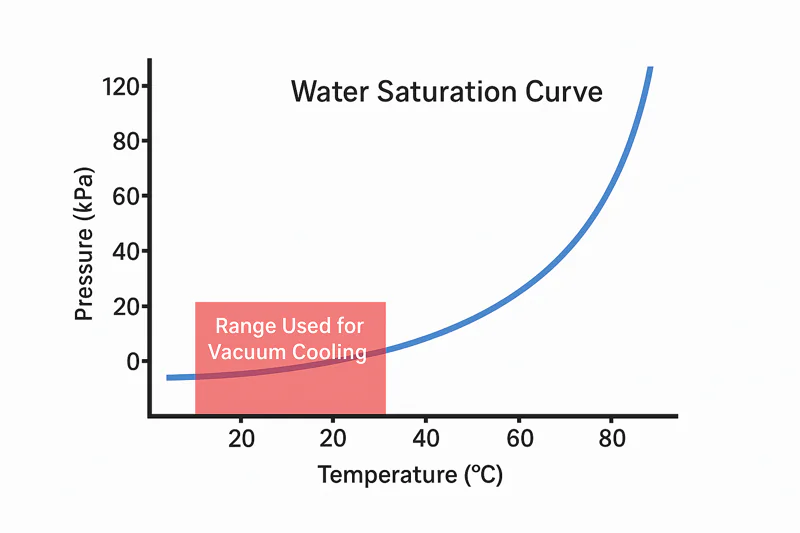
Let’s forget the complex name and think of it as a fixed set of pairs. For every temperature, there is one specific pressure that allows water to boil3 there. It’s a one-to-one relationship.
| If We Want Water to Boil at… | We Must Create This Pressure… |
|---|---|
| 100°C (212°F) | 101.3 kPa (Normal Air Pressure) |
| 20°C (68°F) | 2.3 kPa |
| 5°C (41°F) | 0.87 kPa |
| 2°C (35.6°F) | 0.70 kPa |
This relationship is our guide. When I work with a client like Sophia in her central kitchen, she needs her cooked products cooled to exactly 4°C for HACCP compliance. I don’t have to guess. I program the machine’s control system (the PLC) with that target. The PLC then uses this "map." It tells the vacuum pump to run until the pressure inside the chamber hits exactly 0.81 kPa. It then holds that pressure steady. At that precise pressure, water cannot help but boil at 4°C. The machine uses sensors to continuously monitor the pressure, making tiny adjustments to keep it locked on that target. This turns a complex scientific principle into a reliable, automated, and incredibly precise industrial process.
How Do My Machine’s Gauges Show This Relationship in Action?
Theory is great, but seeing is believing. The control panel on your Allcold vacuum cooler isn’t just a set of numbers; it’s a live dashboard showing you this scientific principle at work in real-time.
On your control panel, you will see two main readings: pressure and temperature. As the machine pulls a vacuum, you will watch the pressure gauge fall. A few moments later, the temperature probe will begin to drop, directly following the pressure down the curve.

Let’s walk through a typical cycle for a pallet of fresh lettuce. First, you close the door and press START.
- The Pump Engages: You’ll hear the vacuum pump4 start. On the screen, you will see the pressure reading (likely in kilopascals, or kPa) start to drop very quickly. It might go from 101 kPa (normal air) down to 10 kPa in just a minute or two. During this time, the product temperature barely changes.
- The "Flash Point": As the pressure continues to fall, it will cross a critical threshold—the boiling point for the current temperature of the lettuce. Suddenly, the water on all the leaf surfaces begins to boil.
- The Cooling Phase5: This is where the magic happens. Once boiling starts, you will see the temperature gauge, which is connected to a probe placed inside a head of lettuce, start to fall rapidly. The pressure gauge will continue its slow descent from around 2 kPa down to under 1 kPa. As it does, the temperature reading will chase it down: 20°C, 15°C, 10°C, 5°C. The two gauges move in a beautiful, predictable dance. The PLC is watching the temperature, and once it hits your target (e.g., 3°C), it stops the cycle. This live feedback is proof that you are not just using a machine; you are commanding a fundamental law of physics.
What Happens When This Pressure-Temperature Link is Broken?
This relationship is a law, but the machine that uses it is subject to real-world issues. If your cooling cycle is slow or fails to reach the target temperature, it’s almost always because the pressure-temperature link has been compromised.
If the machine cannot create and hold the required low pressure, water will never boil at the target low temperature. This is almost always caused by a vacuum leak, which prevents the machine from "hitting its number" on the pressure curve.

This is the most common troubleshooting issue I help my clients with. Think of it like trying to fill a bucket that has a hole in it. The vacuum pump6 is the engine working to remove all the air from the chamber. But if there’s a leak, outside air (at high pressure) is constantly seeping back in. The pump has to fight against this incoming air, and it may never be able to reach the ultra-low pressure target needed for cold boiling. For example, to get to 2°C, you need to hit 0.70 kPa. If a leak prevents your pump from getting the pressure below 1.0 kPa, the absolute coldest your product can ever get is about 7°C, no matter how long you run the machine. This is a point I always stress to hands-on owners like Carlos. Keeping the machine in good order is all about maintaining that perfect seal. The most common sources of leaks are simple and easy to fix:
- The Door Seal7: The large rubber gasket on the door is the most common culprit. If it’s dirty, has debris on it, or is worn out, it won’t create a perfect seal.
- Valves: A valve that is stuck or not closing completely can let air seep back in.
- Pipe Fittings: A loose fitting or a cracked weld in the plumbing can be a small but critical source of a leak.
Understanding this helps you diagnose problems fast. If the temperature stalls, look at the pressure gauge. If it’s not as low as it should be, you have a leak. It’s that simple.
Conclusion
The powerful link between pressure and temperature is the heart of vacuum cooling. It’s a predictable, unbreakable law of physics that you can watch and control directly from the panel of your machine every single day.
-
Understanding atmospheric pressure is crucial for grasping how it influences boiling points, especially in different altitudes. ↩
-
Exploring vacuum coolers can reveal innovative methods for cooking and preserving food by utilizing low pressure. ↩
-
Understanding the pressure-temperature relationship for boiling water is crucial for various scientific and culinary applications. ↩
-
Exploring how vacuum pumps function can enhance your knowledge of food preservation techniques, improving product shelf life. ↩
-
Understanding the Cooling Phase is crucial for optimizing the vacuum cooling process, ensuring freshness and quality of produce. ↩
-
Understanding vacuum pumps is crucial for troubleshooting and ensuring optimal performance in your equipment. ↩
-
Learn how to properly maintain the door seal to prevent leaks and improve the efficiency of your vacuum system. ↩

Mila
You May Also Like

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Why Do Vacuum Cooler Energy Costs Vary So Much?
You’re calculating the return on investment for a new vacuum cooler, but there’s a huge unknown: the electricity bill. You
What Goes Into Designing a High-Quality Vacuum Cooler?
At first glance, a vacuum cooler seems simple: it is a steel box that makes vegetables cold. But this simplicity

What Are the Most Important Features in a Vacuum Cooler?
You’re comparing quotes from different suppliers, and the specification sheets all start to look the same. They all list a

Where Can You Find Reliable Vegetable Vacuum Cooler Suppliers?
You’ve made the decision to invest in a vacuum cooler, but now you face an even bigger challenge: finding a
How Much Does a Vegetable Vacuum Cooler Really Cost?
You know you need a vacuum cooler to improve your product quality, but the price is a huge question mark.

Industrial vs. Commercial Vacuum Coolers: Which Should You Choose?
Your business is growing, and you know that rapid post-harvest cooling is the key to quality and profit. But as

What's the Real Difference Between a High-Quality and a Low-Price Vacuum Cooler?
You are looking for a vacuum cooler, and you see a huge range of prices. One supplier quotes a price

How Do You Choose the Best Vegetable Vacuum Cooler in 2024?
Choosing a vacuum cooler is one of the biggest investments you’ll make in your farm or food business. The market

What Can You Learn from Farms That Mastered Vacuum Cooling?
You see the challenges in your own operation every day: the race against field heat, the constant worry about shelf
