
How Does Vacuum Cooling Actually Make Things Cold?
You’re looking at a pallet of freshly harvested lettuce, warm from the field. You’re told a machine can make it field-fresh and cold in just 25 minutes. It sounds impossible, like something from a science fiction movie.
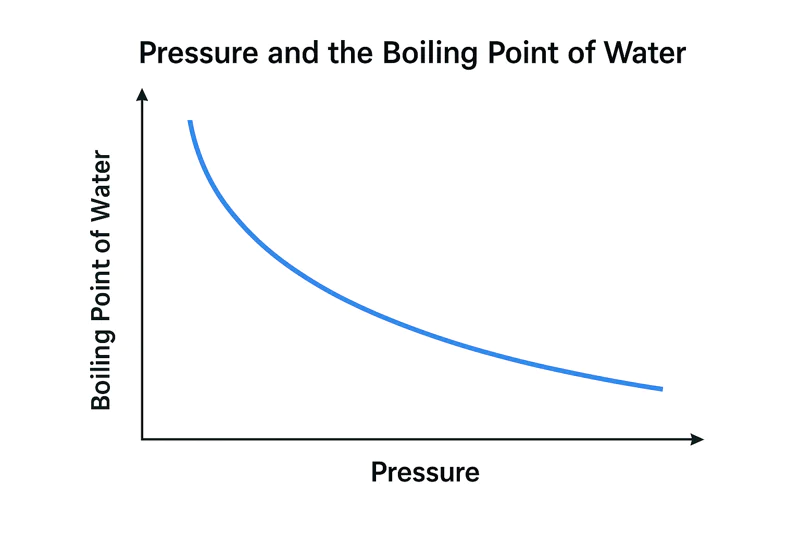
Vacuum cooling works by lowering air pressure. This makes water boil at a very low temperature. This low-temperature boiling pulls heat directly from the product, cooling it rapidly from the inside out. It’s not magic; it’s physics.
This core principle is one of the most powerful tools in modern food preservation. It’s a game-changer for farmers, bakers, and food processors. But to truly appreciate why it’s so effective, you need to understand the simple science behind it. Let’s break down exactly how dropping the pressure can create such a powerful cooling effect.
How Can Water Boil at Room Temperature?
You were taught in school that water boils at 100°C (212°F). This fact makes it hard to understand how we can boil water off a lettuce leaf without cooking it. The secret lies in something we don’t always think about: air pressure.
The boiling point of water is not a fixed number; it depends entirely on the pressure around it. By dramatically lowering the pressure in a vacuum chamber, we can make water boil at just 5°C (41°F).
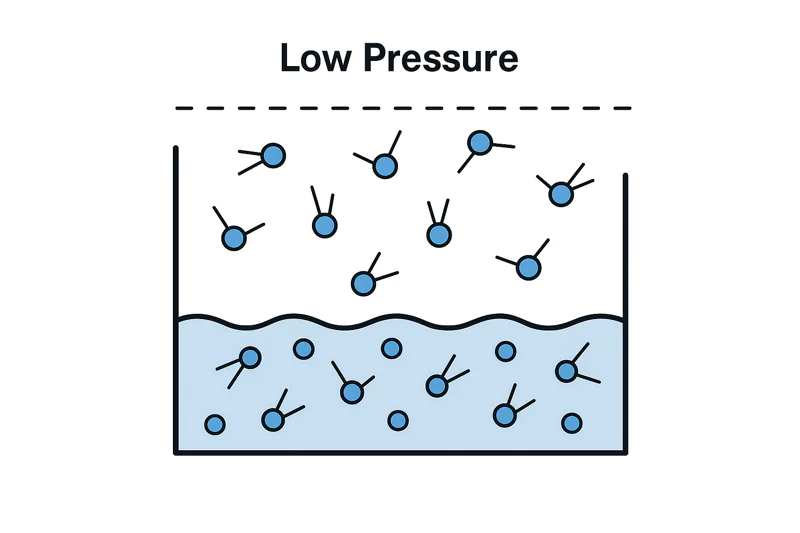
Let’s use a simple analogy. Imagine you are trying to push your way out of a crowded room. If the room is packed (high pressure), it’s very difficult to get out. If the room is nearly empty (low pressure), you can leave with almost no effort. Water molecules are the same. At normal sea-level air pressure, the "pressure" of the air on the water’s surface is high, so the water molecules need a lot of energy—in the form of heat—to escape as steam. This happens at 100°C. But inside a vacuum cooler, we pump almost all the air out. With very little air pressure pushing down on it, the water molecules can escape very easily. They don’t need much energy at all. This means they can "boil" or turn into a gas at a very low temperature. A technically-minded farm owner like Carlos can appreciate this direct relationship.
| Pressure Level | Atmospheric Pressure | Water Boiling Point |
|---|---|---|
| Normal (Sea Level) | 101.3 kPa | 100°C (212°F) |
| High Altitude (Everest) | ~33.7 kPa | ~70°C (158°F) |
| Inside Vacuum Cooler | 0.8 kPa | ~5°C (41°F) |
This is why we can use this process on delicate products. We are not adding heat; we are creating an environment where water can vaporize at a safe, cold temperature.
Where Does the Heat Actually Go During Cooling?
So, the product gets cold fast. But where does the heat energy from the warm lettuce or freshly baked bread go? It doesn’t just vanish into thin air. It gets carried away by the very water that is boiling off the product.
The heat is removed through a process called the "latent heat of vaporization." When water changes from a liquid to a gas (steam), it must absorb a massive amount of energy from its surroundings. This energy is the heat from your product.
Think about why you feel cold after swimming. As the water evaporates from your skin, it pulls heat directly from your body, making you shiver. Vacuum cooling is just a super-fast, powerful version of that same effect. For every one gram of water that we boil off your product, it removes a huge amount of heat energy. This is an incredibly efficient way to transfer heat. Instead of trying to cool the surface slowly with cold air, like in a traditional cold room, we are actively pulling heat from the entire mass of the product at once. This is what allows a vacuum cooler to cool a whole pallet of lettuce from 25°C down to 3°C in under 30 minutes. Of course, there is a trade-off. This evaporation means the product loses a tiny bit of weight, typically around 1% for every 5°C or 10°F of cooling. For a professional buyer like Norman, understanding this predictable and minimal weight loss is key to calculating his final yields. It’s a small price to pay for a massive gain in shelf life and quality.
Why Is Surface Moisture So Important?
You might notice that leafy greens like spinach and lettuce cool down incredibly fast, while denser items like carrots or potatoes don’t respond as well. This isn’t a flaw in the machine; it’s all about the product’s relationship with water.
Vacuum cooling is fueled by evaporation. Therefore, products with a large, moist surface area cool much more effectively because they provide more fuel (water) for the process. Think of it as having more places for the "boiling" to happen.

The key factor here is the surface-area-to-mass ratio. A head of iceberg lettuce is a perfect example. It’s made of many thin, wet leaves, giving it a gigantic surface area relative to its total weight. This provides endless locations for the low-temperature boiling to take place, so the cooling is extremely rapid and uniform. A potato, on the other hand, is dense. It has a very small surface area for its large mass, and its skin prevents moisture from escaping easily. This is a critical point I always discuss with new clients. For a central kitchen manager like Sophia, who needs to cool cooked pasta or rice, we know the product has plenty of free moisture. But for a vegetable grower like Carlos who wants to cool broccoli, which is denser than lettuce, we might recommend a solution. We can add a "hydro-vac" feature to our systems. This gives the produce a quick, gentle shower before the cycle starts, ensuring there is enough surface moisture to fuel a fast and effective cooling cycle. Understanding this principle lets you optimize the process for every type of product.
Product Suitability for Vacuum Cooling
| Category | Examples | Why it Works |
|---|---|---|
| Excellent | Lettuce, Spinach, Mushrooms, Cabbage | High surface area, high natural moisture.1 |
| Good | Broccoli, Cauliflower, Celery, Sweet Corn | Good moisture content, benefits from pre-wetting. |
| Challenging | Potatoes, Onions, Carrots, Tomatoes | Low surface area, protective skin prevents evaporation. |
| Cooked Foods | Rice, Pasta, Stews, Baked Bread | High free moisture, excellent for rapid cooling.2 |
What Key Components Make This Science Work?
Knowing the physics is great, but how does a big steel box actually put these principles into practice? The machine itself is a coordinated system, where four main components work together to create the right conditions for rapid cooling.
A vacuum cooler is a system built around four pillars: the Chamber to seal the product, the Pump to remove the air, the Refrigeration unit to trap the water vapor, and the Controls to manage the process.
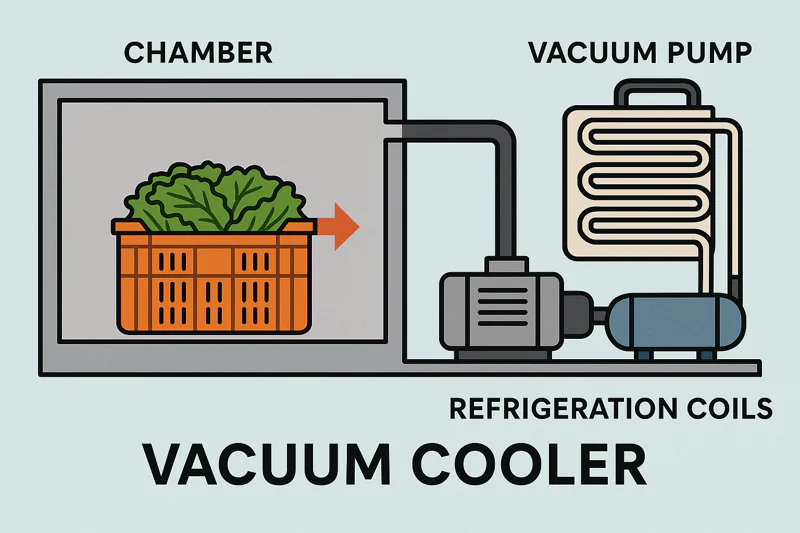
Let’s look at the job of each component. Thinking about how they work as a team makes the whole machine much easier to understand.
The Vacuum Chamber
This is the most visible part—the strong, stainless steel3 box with a heavy door. Its only job is to be a container that can be sealed perfectly airtight4. It must be strong enough to withstand the immense force of the outside air pressure trying to crush it when the near-vacuum is created inside. We use high-quality stainless steel because it’s durable and, critically for food-grade applications, easy to clean and sanitize.
The Vacuum Pump5
This is the engine of the system. Its job is to pump air out of the sealed chamber, creating the low-pressure environment6 we need. The size and power of the pump determine how quickly the chamber can reach the target pressure, which influences the overall cycle time. It’s the workhorse that makes the low-temperature boiling possible.
The Refrigeration System (Vapor Trap)
This is the most misunderstood component. The refrigeration coils7 inside the chamber do not cool your product directly. Their job is to get extremely cold, like a block of ice. As the water from your product boils and becomes a gas (water vapor), this vapor is drawn towards the ice-cold coils and instantly freezes onto them. We call this the "vapor trap8" or "ice catcher." This is essential because it removes the water vapor from the chamber, allowing the pump to maintain the low pressure needed to keep the process going.
The Control System (PLC)
This is the brain. The Programmable Logic Controller (PLC)9 is a small computer that runs the entire cycle. It takes readings from pressure and temperature sensors and tells the pump and refrigeration system when to turn on and off. It ensures the cooling curve10 is perfect for your specific product, guaranteeing a consistent result every single time.
Conclusion
Understanding this science shows that vacuum cooling isn’t magic. It is a predictable and powerful tool, harnessing basic physics to give you unparalleled control over the quality and shelf life of your products.
-
Understanding these benefits can enhance your knowledge of effective cooling methods for various produce. ↩
-
Exploring this topic can provide insights into optimizing cooling processes for better food preservation. ↩
-
Explore this link to understand why stainless steel is preferred for vacuum chambers, especially in food-grade applications. ↩
-
Discover techniques and tips for achieving an airtight seal, crucial for effective vacuum chamber operation. ↩
-
Understanding the mechanics of a Vacuum Pump can enhance your knowledge of its applications and efficiency. ↩
-
Exploring the concept of a low-pressure environment can provide insights into its importance in various scientific and industrial processes. ↩
-
Exploring how refrigeration coils function can enhance your knowledge of cooling technology and its applications. ↩
-
Understanding the vapor trap is crucial for optimizing refrigeration efficiency and product preservation. ↩
-
Understanding PLCs is crucial for optimizing automation processes in various industries. ↩
-
Exploring the cooling curve can enhance your knowledge of product consistency and quality control. ↩

Mila
You May Also Like

How Do You Guarantee a Perfect Cooling Cycle Every Single Time?
You’ve invested in a state-of-the-art vacuum cooler, but its performance depends entirely on the people who use it every day.

Why Do Vacuum Cooler Energy Costs Vary So Much?
You’re calculating the return on investment for a new vacuum cooler, but there’s a huge unknown: the electricity bill. You
What Goes Into Designing a High-Quality Vacuum Cooler?
At first glance, a vacuum cooler seems simple: it is a steel box that makes vegetables cold. But this simplicity

What Are the Most Important Features in a Vacuum Cooler?
You’re comparing quotes from different suppliers, and the specification sheets all start to look the same. They all list a

Where Can You Find Reliable Vegetable Vacuum Cooler Suppliers?
You’ve made the decision to invest in a vacuum cooler, but now you face an even bigger challenge: finding a
How Much Does a Vegetable Vacuum Cooler Really Cost?
You know you need a vacuum cooler to improve your product quality, but the price is a huge question mark.

Industrial vs. Commercial Vacuum Coolers: Which Should You Choose?
Your business is growing, and you know that rapid post-harvest cooling is the key to quality and profit. But as

What's the Real Difference Between a High-Quality and a Low-Price Vacuum Cooler?
You are looking for a vacuum cooler, and you see a huge range of prices. One supplier quotes a price

How Do You Choose the Best Vegetable Vacuum Cooler in 2024?
Choosing a vacuum cooler is one of the biggest investments you’ll make in your farm or food business. The market

What Can You Learn from Farms That Mastered Vacuum Cooling?
You see the challenges in your own operation every day: the race against field heat, the constant worry about shelf
